Metallic bond-

source : You tube
Metallic bond-
There are two models to explain presence of metallic bond in metals-
1) Electron sea model or Free electron model-
Electron sea model was proposed by Drude & later on developed by Lorentz.This model is based upon the following properties of metals-
a) Presence of empty orbitals –
In metals number of valence electrons is less than number of valence orbitals. Hence large number of orbitals in valence shell are empty.
Ex. 3Li – 1s2,2s1
In Li , 2p orbitals are vacant.
11Na – 1s2,2s2 2p6,3s1
In Na(sodium) , 3p & 3d orbitals are vacant.
b) Low Ionization energy-
The valence electrons of metal atoms are not strongly held by the nucleus . So metals have low ionization energies. Remaining part of atoms after losing electrons is called Kernels. Kernels are nuclei with inner shell electrons.
Ex – Li+,Na+, Al3+ are kernels.
The important postulates of electron sea model are –
i) The atom constituting a metal possess many vacant orbitals in their valence shell.
Ex. 3Li – 1s2,2s1
second shell is valence shell . In valence shell vacant 2p orbitals are present.
12 Mg – 1s2,2s2, 2p6, 3s2
Third shell is valence shell . In this shell vacant 3p & 3d orbitals are present.
ii) Metals possess comparatively low value of ionization energy. Due to low ionization energy, metal has a tendency to lose valence electrons easily & to form positive ion called Kernel. The kernel consists of metal nucleus & inner shell electrons.
Ex. 3Li – 1s2,2s1
(Kernel) Li+ – 1s2
Li+ (Kernel ) consists of Lithium nucleus & 1s2 electrons.
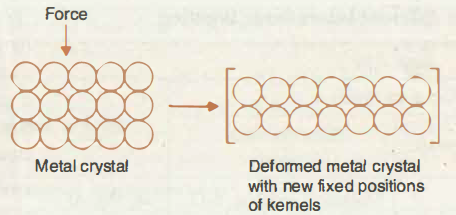
iii) The Kernels occupy fixed positions called lattice sites. The electrons lost by the atoms are not attached to any kernel but are free to move like the molecules of gas through out the space between the kernels. Thus electrons spread all over the available space in the form of a charge cloud. Such electrons are called delocalized.
Thus a metal can be composed of an assembly of positive ions immersed in a sea of electrons. Therefore this model is called ‘Electron sea model’.
iv) Atoms in a metal are closely packed.Due to close packing of atoms, several electron charge clouds are attracted by several kernels. So each kernel comes nearer to several electron charge clouds.This leads to a decrease in the potential energy of the system & metal atoms get attached to one another by strong cohesive forces.
Thus the force of attraction between the mobile electrons & the kernels is known as metallic bond . It is non directional & weaker than covalent bond.