Normality, Molarity,Molality

source :khan academy
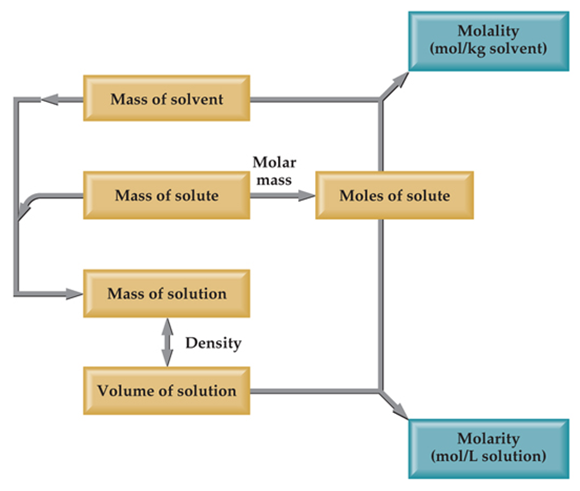
Molality :
“Number of moles of solute present in one kg. of solvent is called molality “.
Molality = Number of moles of solute/weight of solvent(Kg.)
Number of moles of solute = w/m =weight of solute/molecular weight of solute
=w/m W(Kg.)
Deci molal = When 1/10 moles of solute are present in one kg. of solvent ,then solution is deci molal.
Centi molal = When 1/100 moles of solute are present in one Kg. of solvent ,then solution is centi molal.
milli molal = When 1/1000 moles of solute are present in one Kg. of solvent then solution is milli molal.
Mole fraction:
” Number of moles of the substance divided by total number of moles of Solution is called mole fraction “.
If three components A, B & C are present,
then Number of moles of A = nA
Number of moles of B = nB
Number of moles of C = nC
Total no. of moles = nA+nB+nC
Mole fraction of A = nA/(nA + nB+ nC)
XA = (wA/mA) /[ (wA/mA)+(wB/mB)+(wC/mC)]
XB =(wB/mB) /[ (wA/mA)+(wB/mB)+(wC/mC)]
XC = (wC/mC) /[ (wA/mA)+(wB/mB)+(wC/mC)]
(XA+XB+XC = 1) mole fraction is always unity.
Concentration in ppm (parts per million) –
“It is equal to the number of milligrams of the solute present in one liter of solution .”
concentration in ppm =weight of solute (mg.)/ volume of solution (l)
Formality :
“Formality is equal to the number of formula masses present in one liter of the Solution.”
F = number of formula masses of solute / Volume of solution (L)
F = weight of solute in gm. / (formula mass of solute x volume of solution in liter)
It is used to express the concentration of ionic components such as NaCl , KBr , KI etc.
Q. 7.45 gm of KCl is dissolved in 100 gm of H2O. calculate mole fraction of KCl ?
Solution :
m of KCl =39 +35.5 =74.5
moles of KCl =w/m= 7.45 /74.5 =0.1
moles of H2O =w/m =100/18 = 5.55
total moles =0.1 +5.55 =5.65
mole fraction of KCl= moles of KCl / total moles
0.1 /5.65 =0 .018
mole fraction of KCl =0.018 ans.
Q. A sugar syrup of weight 214.2 gm. contains 34.2 gm. of sugar. Calculate (i) Molality or Molal Concentration. (ii) Mole fraction of sugar & water in the syrup?
Solution:
molecular weight of sugar = 342
w = 34.2 gm.
Weight of solvent= 214.2-34.2
= 180 gm
= 0.180 kg.
Molality = w/ m W(Kg.)
=34.2/ 342 x 0.180
Molality = 0.56 mole /Kg.
no. of moles of sugar (ns) =w/m 34.2/342
= 0.1
Moles of water nw = w/m = 180/18 = 10
Total moles= 10 +0.1 =10.1
Xs = ns/total moles
=0.1/10.1= 0.0099
mole fraction of sugar =0.0099
Xw = nw / total moles
=10/10.1 =0.990
mole fraction of water =0.990