Order of reaction-

source : Youtube
Order of reaction-
Q 1) Starting with one mole of a compound ,it is found 3/4 of the reaction is completed in one hour . Calculate the rate constant if the reaction is of (i) first order (ii) second order
Solution )
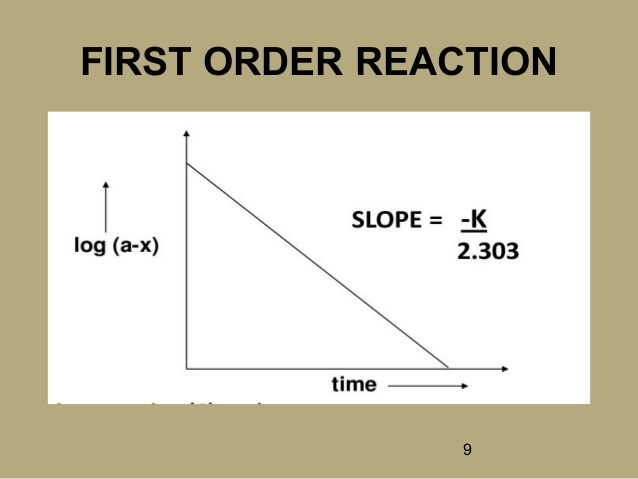
i) For first order reaction-
t = 1 hour , a =1 mole, x = 3/4 mole
K= 2.303 / t [log (a/a-x)]
K = 2.303 / 1 [log 1 /( 1 – 3/4)]
K = 2.303 [log 1 /( 1/4)]
K = 2.303 (log 4)
log 4 = 0.6020
K = 2.303 x 0.6020
K = 1.386 hour-1 Ans
ii) For second order reaction –
a=1 mole , x = 3/4 mole , t = 1 hour
K =1/t [x/a(a-x)]
K =1/1 [(3/4)/1(1 – 3/4)]
K = 1/ 1 [ (3/4)/( 1/4)
K = 3.0 litre mole-1 hour-1 Ans.
Q 2) A second order reaction in which both the reactants have same concentration , is 20 % completed in 500 sec. How much time will it take for 60 % completion ?
Solution )
a=100 , x = 20, t = 500 sec.
K =1/t [x/a(a-x)]
K = 1/ 500 [20/100 (100 – 20)
K = 1/ 500 (20/ 100 x 80)
K=20 / 500 x 100 x 80
K = 5 x 10-6 litre mole-1 sec-1
a = 100 , x = 60
t =1/K [x/a(a-x)]
t = 1/ 5 x 10 -6 [60/100 (100 – 60)
t = 1/ 5 x 10 -6 (60/ 100 x 40)
t = 60 / 5 x 10 -6 x 100 x 40
t = 3000 sec. Ans.
Q 3) The decomposition of N2O5 in CCl4 solution has been found to be first order with respect to N2O5 with rate constant K = 6.2 x 10 -4 sec-1
N2O5 ——-> 2 NO2 + 1/2 O2
Calculate the rate of reaction when ,
(i) [N2O5] = 2.5 mole / litre
(ii) [N2O5] = 0.50 mole / litre
(iii) What concentration of N2O5 would give a rate of 4.2 x 10 -3 mole litre-1sec-1.
Solution-
(i) K = 6.2 x 10 -4 sec-1 ,
[N2O5] = 2.5 mole / litre
Rate = K [N2O5] = 6.2 x 10 -4 x 2.5
Rate =1.55 x 10-3 mole litre-1sec-1 Ans
(ii)
K = 6.2 x 10 -4 sec-1 ,
[N2O5] = 0.50 mole / litre
Rate = K [N2O5] = 6.2 x 10 -4 x 0.50
Rate = 3.1 x 10-4 mole litre-1sec-1 Ans
(iii) Rate = 4.2 x 10 -3 mole litre-1sec-1
K = 6.2 x 10 -4 sec-1 ,
[N2O5] = Rate / K
= 4.2 x 10 -3/ 6.2 x 10 -4
Rate = 6.77 mole / litre Ans.
Q 4- find the order of reaction for the rate expression , Rate = K[A] [B] 2/3 . Also suggest the units of rate and rate constant for this expression.
Solution –
Rate = K[A] [B] 2/3
order of reaction = 1 + 2/3
order of reaction = 1.67 Ans.
Unit of rate –
Rate = dx/dt= (mole/litre)/ time
unit of rate = mole litre-1 time -1 Ans.
Unit of rate constant –
Rate = K [A] [B]2/3
K = (mole litre-1 time -1)/ (mole litre-1)(mole litre-1)2/3