oxidation & reduction

source : www.layers-of-learning.com
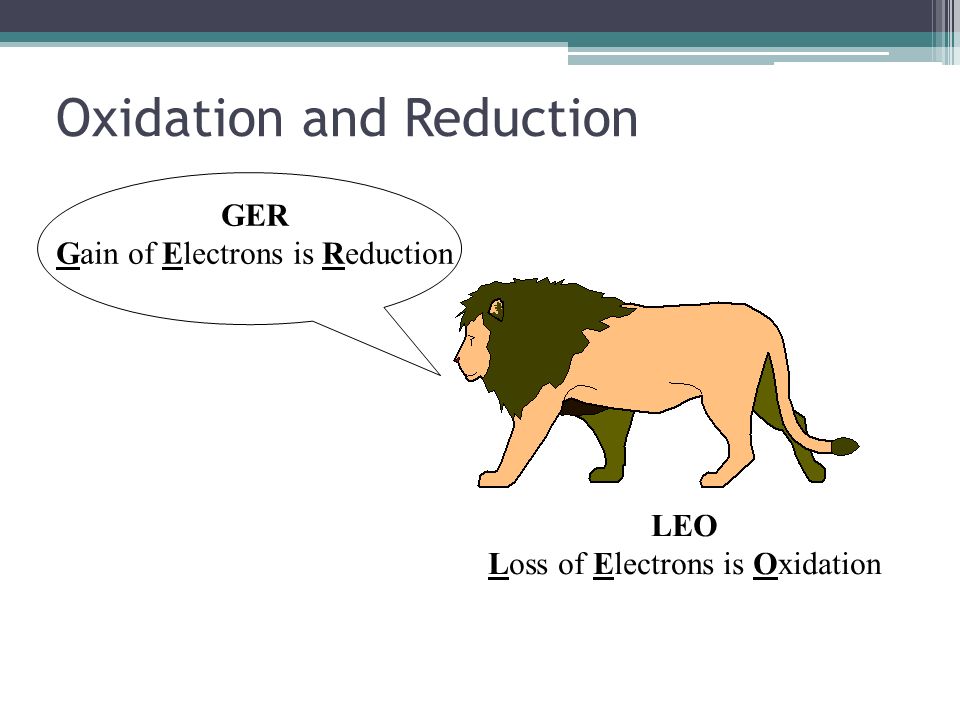
Oxidation
Oxidation or de-electronation is a process which releases electrons. In oxidation, oxidation number increases
Ex. Na—–> Na++e
Cu——-> Cu++
Al ——-> Al+++
Ca ——->Ca++
Reduction
Reduction is a process which gains electron. In reduction, oxidation number decreases
O+2e —–> O– –
F +e ——->F–
Cl +e ——>Cl–

source : texampe.net

source : chemistry tutor vista.com