oxidising & reducing agents

source : slide player.com
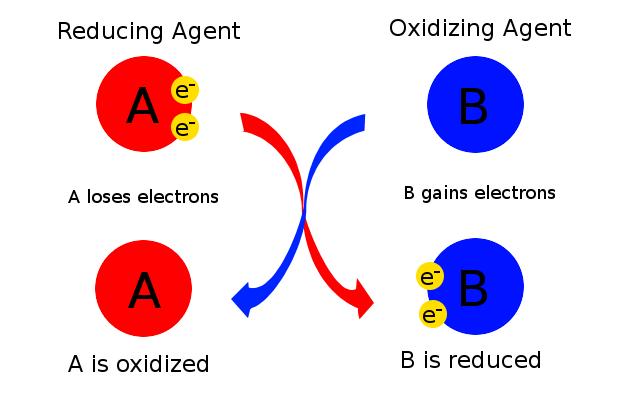
Oxidising agents or oxidants : are substances which
- Show gain of electrons
- Oxidise other substances
- are reduced themselves
- Show a decrease in oxidation no.
Reducing Agents or Reductants : are substances which
- Show loss of electrons
- reduce other substances
- are oxidised themselves
- show an increase in oxidation no.
Redox reaction is a process in which reducing agent is oxidised & liberate electrons, which are then taken up by an oxidising agent to get itself reduced. In redox rxns oxidation and reduction occur simultaneously.
Ex.
- Identify the oxidizing agent and the reducing agent in the following redox reaction:
MnO2(s)+4H+(aq)+2Cl−(aq)→Mn2+(aq)+2H2O(l)+Cl2(g)
- Cl−
is the reducing agent because it is oxidized and loses one electron (starting with an oxidation state of -1 in the Cl− ions and increasing to 0 in Cl2). Remember that gaining electrons means it is “reduced”. MnO2 is the oxidizing agent because it is reduced by gaining two electrons (starting with Mn in an oxidation state of +4 in MnO2 and decreasing to +2 in free Mn2+
- ions). Keep in mind that losing electrons means it is “oxidized”.