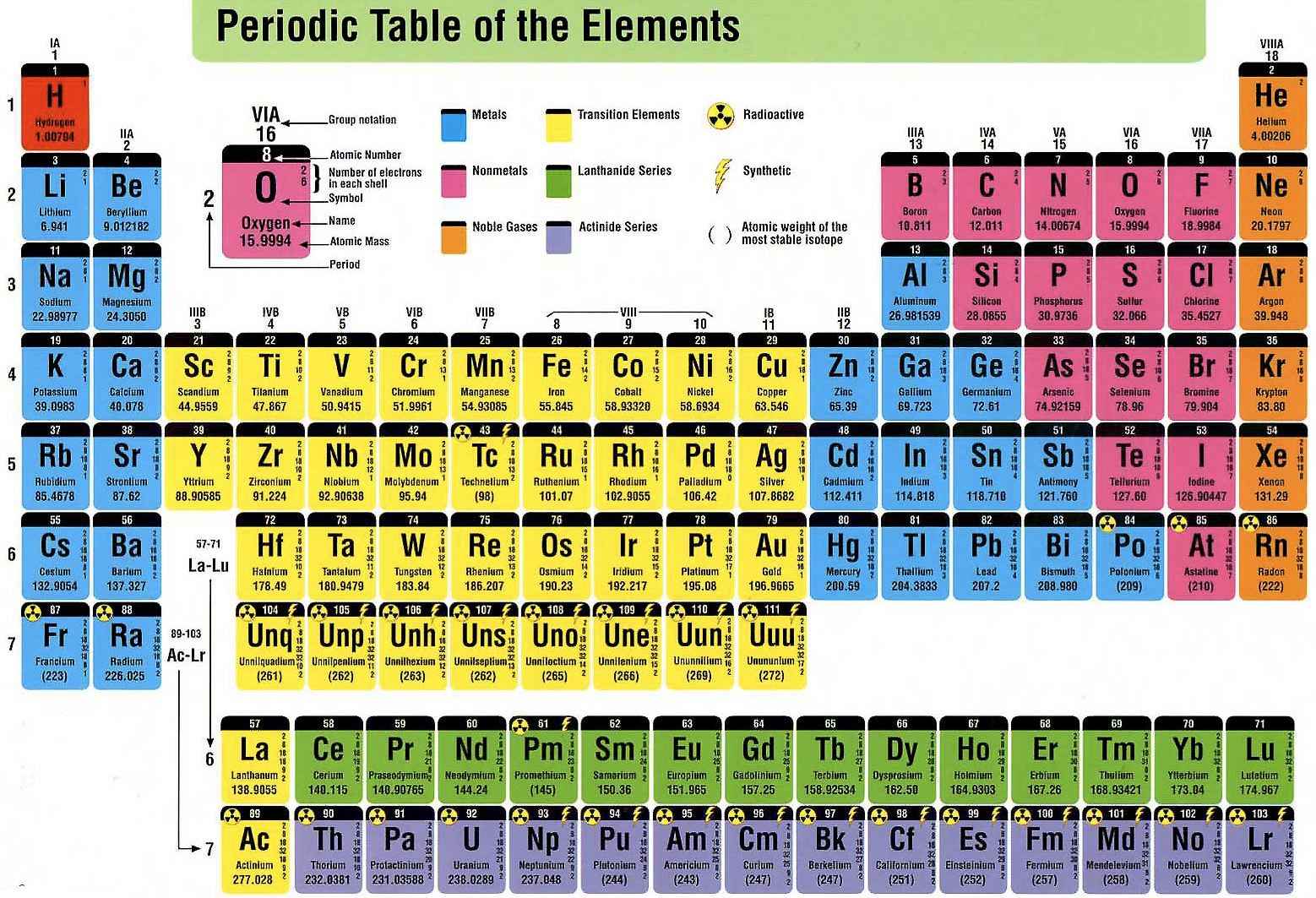
Modern periodic table
source : youtube
Modern Periodic law –
Mendeleef’s periodic law was modified because atomic mass is not the fundamental property of element , Mosley gave the modern periodic law, according to this law,
” The physical & chemical properties of the elements are periodic function of their atomic numbers.”

source : SlidePlayer
In Mendeleeff ‘s modern periodic table , elements have been arranged in the increasing order of their atomic numbers.With the replacement of atomic mass by atomic number as the basis of classification, two main defects of original periodic table disappears. These are,
1) Anamalous pair –
The original periodic law was violated in case of 4 pairs of elements . The element of higher atomic weight was placed before the elements having lower atomic mass.
Ex- Ar K ; Co Ni ; Te I ; Th Pa
At. Mass 40 39 ; 60 58.6 ; 127.5 127 ; 232 231
At. No. 18 19 ; 27 28 ; 52 53 ; 90 91
This problem is removed when elements are arranged in order of increasing atomic number.
2) Position of isotopes –
Atoms having same atomic number but different atomic masses are called isotopes .
The isotopes of same element will be given different position if atomic mass is the basis of classification. In modern periodic table isotopes are placed in one position. So this problem is removed.
Characteristics of Modern periodic table-
1) The modern periodic table consists of nine vertical columns called groups & seven horizontal rows called periods.
2) Groups –
a) There are 9 Groups ( including zero group) in periodic table. In zero group inert gases are present .This group is present in the extreme right side of the periodic table.
b) Except eighth & zero group , all are divided into two subgroups. These are sub group A & sub group B. The elements of sub group A have been placed in the left side of the periodic table & elements of sub group B at right side of the periodic table in the same group. In eighth group elements are present in three columns. Like,
viii viii viii
Fe Co Ni
Ru Rh Pd
Os Ir Pt
c) Elements belonging to the same group are similar in properties but elements of same subgroup are more close in their properties.
Periods –
a) Modern periodic table contains 7 periods. In first period only two elements are present.
b) The second & third period contain 8 elements each.
First period 2 elements Very short period
second period 8 elements short period
Third period 8 elements short period
Fourth period 18 elements long period
Fifth period 18 elements long period
Sixth period 32 elements Very long period
seventh period 23 elements incomplete period
c) In sixth period , out of 32 elements , 14 elements have been placed separately in horizontal row below periodic table . Symmetry of periodic table is destroyed if these 14 elements are placed inside the periodic table.These elements are called lanthanides.
d) In viii th period 23 elements are present. Out of 23 elements, 14 elements (at. No. 90 – 103) are placed below periodic table in a horizontal row. These are called actinides
e) The elements beyond 92 U do not occur in nature. These are prepared by artificial methods & are Called transuranic or posturanic or post uranium elements.
Advantages of Modern Periodic table –
1) In the study of elements & their compounds –
A general study of these 9 groups give some idea about properties of 109 elements & their compound . So with the help of periodic table systematic study of elements & their compounds become easy.
2) Correction of atomic weights of elements –
The position of elements in the periodic table gives information about its valency & atomic weight. Atomic weight of Pt & U were corrected.
3) Correction of the valency of elements-
At.mass = Eq.wt. x valency
eq.wt. of Be= 4.5 , valency =3
at.wt. = 4.5 x 3= 13.5
So Be should be placed between C12 & N14, but it resembles second group elements & no space vacant between C & N. So Be is given proper place by correcting its valency i-e. 2.
at.mass = 4.5 x 2 = 9.0
4) Discovery of new elements –
In periodic table many places were left vacant because no suitable element was known at that time. Now those all elements have been discovered for which Mendeleeff left space. Their properties have been found same as predicted by Mendeleeff.
Defects in Modern Periodic table –
1) Place of hydrogen –
Hydrogen is placed at two places i-e. in both first (with alkali metals) & viii th group ( with halogens) . Due to its resemblance with alkali metals & halogens.
2) Dissimilar elements placed together –
In first group highly reactive alkali metals ( Li, Na, K) & less reactive metals ( Cu, Ag, Au ) have been placed in different groups. Some elements are similar in properties but they have been placed in different groups.Like, Ba & Pb ; Ag & Tl
3) Position of lanthanides & actinides –
Lanthanides (14 elements from 58Ce – 71 Lu ) have been placed below the periodic table in a horizontal row
( Elements of third group & sixth period ).
Actinides (14 elements from 90 Th – 103 Lr ) have been placed below the periodic table in a horizontal row ( Elements of third group & seventh period ).