Prediction:Hybridization
source : ck 12.org
Prediction of Hybridization Geometry (shape) of Covalent Molecules :
There are two method :
First Method:
H = number of sigma bond + number of lone pair of electrons at that atom
If value of ‘H’ is two then sp , three then sp2, four then sp3.
Example: 1 ) BeCl2
Number of sigma bond at Be atom = 2
Number of lone pair of electrons at Be atom = 0
H = number of sigma bond + number of lone pair electrons at that atom
=2+0 =2 (sp hybridization, angle 180 degree and geometry of molecule is linear)
Example: 2) CCl4
Number of sigma bond = 4
Number of lone pair of electrons at C- atom = 0
H = number of sigma bond + number of lone pair of electrons at that atom
=4+0 =4 (sp3 hybridization, angle 109o28‘ and shape of molecule is Tetra hedral)
Example 3: NH3
number of sigma bond = 3
number of lone pair of electrons at N-atom = 1
H = number of sigma bond + number of lone pair electrons at that atom
=3+1 =4 ( sp3 hybridization , angle 109 o28‘ and geometry is Tetra hedral)
Example: 4) H2O
number of sigma bond = 2
number of lone pair of electrons at O-atom = 2
H = number of sigma bond + number of lone pair electrons at that atom
=2+2 =4 (sp3 hybridization, angle 109 o28‘ and shape is Tetra hedral)
Prediction:Hybridization
Alternate Method :
H= [ (V+M – C +A)]/2
H = number of orbitals involved in hybridization
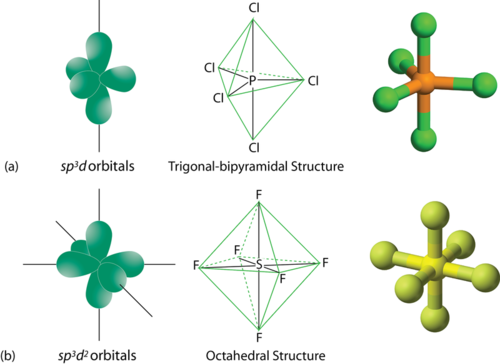
if H = 2 then sp , if H = 3 then sp2, if H = 4 then sp3, if H = 5 then sp3d, if H = 6 then sp3d2
V = Number of electrons in valence shell of central atom.
M = Number of mono-valent atoms
C = Charge on cation
A = Charge on anion
Example:1) NH4+
H= [ (V+M – C +A)]/2
V= 5 ; M =4 ; C=1; A =0
H = (5 +4 -1 +0)/2
=4 (sp3 hybridization)
Example: 2) PO43-
H= [ (V+M – C +A)]/2
V= 5 ; M =0 (because oxygen is divalent ) ; C=0; A =3
H = (5 +0-0 +3)/2
=4 (sp3 hybridization)
Example: 3) SO4 2-
H= [ (V+M – C +A)]/2
V= 6 ; M =0 (because oxygen is divalent ) ; C=0; A =2
H = (6 +0-0 +2)/2
=4 (sp3 hybridization)
Example: 4) SF6
H= [ (V+M – C +A)]/2
V= 6 ; M =6 ; C=0; A =0
H = (6 +6 – 0 +0)/2
=6 (sp3d2 hybridization)
Example: 5) PCl5
H= [ (V+M – C +A)]/2
V= 5 ; M =5 ; C=0; A =0
H = (5 +5 -0 +0)/2
=5 (sp3d hybridization)
V= 6 ; M =0 ; C=0; A =2
Read more articles at chemistryonline.guru