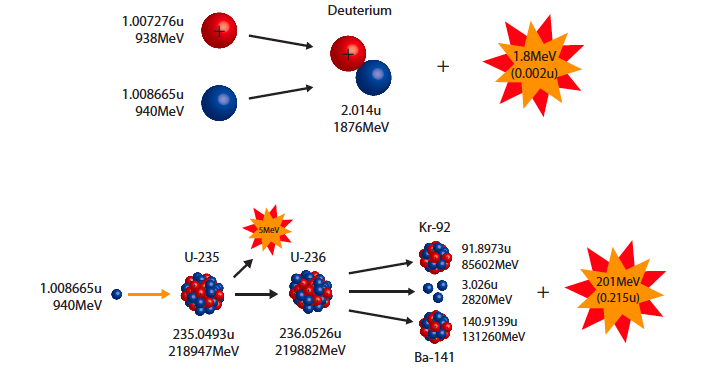
binding energy & mass defect
binding energy &mass defect

source : dc.edu.au
Q 1—Atomic mass of 8O16 is 16.
Mass of one neutron =1.00893 amu
Mass of one proton =1.00757 amu
Mass of one electron =0.0005486 amu
Calculate its mass defect & binding energy?
8O16 have 8p, 8n & 8e
Ans. Mass of nucleus = Mass of 8p +mass of 8n
= 8×1.00757 + 8×1.00893
= 8.06056+8.07144
= 16.1320 a.m.u
Mass of nucleus = Atomic Mass-Mass of 8e
= 16–8×0.0005486
= 16–0.0043888
= 15.9956112 amu
Mass defect (Δm) = 16.1320–15.9956
(Δm) = 0.1364 amu Ans.
Binding energy ( B) = Δm ×931 MeV
= 0.1364×931
B = 126.988 MeV
Q 2—Calculate Δm & B. energy of 33As75. The mass of one proton, one neutron & one electron & atomic mass of 33As75 is 1.0073 amu, 1.0087 amu& 0.0055 amu and 74.9216 amu
Ans. 33As75 = 33e, 33p&42n
Mass of nucleus = mass of 33p + mass of 42n
= 33×1.0073+42×1.0087
= 33.2409 +42.3654
= 75.6063 amu
Mass of nucleus = Atomic Mass-Mass of 33e
= 74.9216 –33×0.00055
= 74.9216- 0.01815
= 74.90345 amu
Δm = 75.6063–74.90345
Δm = 0.70285 amu
B= Δm×931 MeV
= 0.70285×931
B = 654.35 MeV Ans
Q 3 —Calculate the Δm & b. energy of 2He4. Its actual atomic mass is 4.0039 amu. The mass of one n& one p together is 2.0165 amu. mass one electron=0.0005486amu
Ans . 2He4= 2e, 2p&2n
Mass of Nucleus = at. mass–mass of 2e
= 4.0039–2×0.0005486
= 4.0028 amu
Mass of nucleus = mass of 2p +mass of 2n
= 2(mass of P+mass of n)
= 2×2.0165
= 4.033 amu
Mass defect (Δm) = 0.0302 amu An B = Δm×931 MeV
= 0.0302×931
= 28.12 MeV.
Q 4—Binding energy per nucleon for an element is 7.14 MeV. If the binding energy of the element is 28.6MeVCalculate the number of nucleons in the nucleus.
Ans. No. of nucleons =?
B = 28.6MeV
Binding Energy per Nucleon = 7.14MeV
No. of nucleons = =4 Ans.
Q 5—B. energy of an element is 64 MeV. Binding energy per nucleon is 6.39 MeV. What is the total number of neutrons and protons in the nucleus?
Ans. No. of nucleons = No. of Protons + No. of Neutrons
B= 64MeV
No. of Nucleons = =10 Ans
Total no. of neutrons & protons = 10 Ans.
Q 6—B. energy of 2He4 is 28.8 MeV. Calculate Binding energy per nucleon?
Ans. B = 28.8 MeV
No. of Nucleons in 2He4 =4
Binding energy per nucleon =
= = 7.2 MeV
Ans =7.2 MeV