Redox-reaction

source:chemistry.tutorvista.com
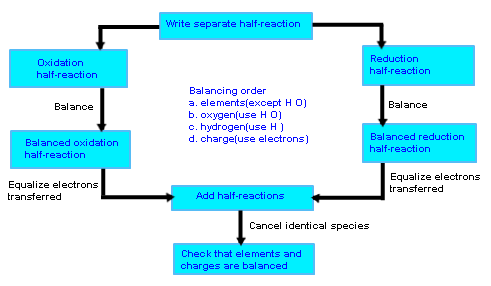
- Balance the Given redox-reaction by ion-electron Method
Cr2O7– – +Fe+++ H+ —-> Cr3++Fe3++H2O
+6
Cr2O7– – + Fe++ + H+ —-> Cr3+ +Fe3+ +H2O
+6
Cr2O7– – ……> Cr3+ (Reduction)
Fe2+ —-> Fe3+ (Oxidation)
Balance reduction half rxn
Cr2O7– – —–> 2Cr3+
To balance oxygen atom
Cr2O7– – —–> 2Cr3++7H2O
To balance H-atom
Cr2O7– – + 14H+ —–> 2Cr3+ + 7H2O
To balance charge
Cr2O7– – + 14H+ 6e– —–> 2Cr3+ + 7H2O …… Eq. (1)
To balance oxidation half rxn-
Fe++ —–>Fe3+
To balance charge
Fe++ —–>Fe+++ + e– …… Eq. (2)
Multiply Eq. (2) by 6 & add Eq (i) & (ii)
6Fe2+ —–> 6Fe3+ + 6e
Cr2O7– – + 6Fe++ + 14H+ —–> 2Cr3++6Fe3+ +7H2O
-
MnO4– + Fe++ + H+→ Mn++ + Fe3++H2O
+7
MnO4– + Fe++ + H+ →Mn++ + Fe3++H2O
+7 +2
MnO4– → Mn++ (Reduction)
To balance reduction half rxn-
MnO4– → Mn++
To balance O-atom
MnO4– → Mn+++ 4H2O
To balance H-atom
MnO4–+8H+→ Mn+++4H2O
To balance charge
MnO4– + 8H+ +5e– → Mn+++4H2O (i)
To balance oxidation half rxn
Fe++ →Fe+++ (Oxidation)
To balance charge
Fe++ → Fe+++ +e (2)
Multiply Eq.(2) by 5 and add both equations
5Fe++ → 5Fe3+ + 5e
MnO4– +5Fe2++8H+ → Mn+++5Fe3+ + 4H2O
D MnO4– + SO3– – → Mn2+ + SO42–
+7 +4 +2 +6
MnO4– + SO3– – → Mn++ + SO4– –
+7 +2
MnO4– – → Mn++ (Reduction)
+4 +6
SO3– – → SO4– – (Oxidation)
To balance reduction half rxn
MnO4– →Mn++
To balance O-atom
MnO4– → Mn++ + 4H2O
To Balance H- atom
MnO4– + 8H+ → Mn++ + 4H2O
To balance Charge
MnO4– + 8H+ + 5e– → Mn++ + 4H2O (i)
To balance oxidation Half Rxn
SO3– – → SO4– –
To balance H-atom
SO3– – +H2O → SO4– – + 2H+
To balance Charge
SO3– – + H2O → SO4– – + 2H+ + 2e (ii)
Multiply eq(1) by 2 and eq. (2) by 5 & add both equations
2MnO4– + 16H+ +10e– → 2Mn+++ 8H2O
5SO3– – + 5H2O → 5SO4– – +10 H+ + 10e–
2MnO4– +5SO3– – + 6H+ → 2Mn++ + 5SO4– – + 3H2O
To balance the given redox-reaction
- NO3– +I– → NO + I
NO3– → NO (Reduction)
-1 0
I – → I2 (Oxidation)
To balance Reduction half rxn-
NO3–→ NO
To balance O-atom
NO3– → NO+2H2O
To balance H-atom
NO3– +4H+ → NO + 2H2O
To balance charge
NO3– + 4H+ + 3e– → NO+2H2O (i)
To balance oxidation half rxn
I– → I2
To balance I-atom
2I– → I2
To balance Charge
2I– → I2 + 2e (ii)
Multiply eq (1) by 2 & eq. (2) by 3 & add both equations
2NO3– + 8H++6e → 2NO+4H2O
6I– → 3I2 + 6e–
2NO3– + 6I– + 8H+ → 2NO + 3I2 + 4H2O
To balance the given redox-reaction
Cr2O7– – + SO2 → Cr3+ + HSO4–
+6
Cr2O7– – → Cr3+ (reduciton)
+4 +6
SO2 → HSO4– (Oxidation)
To balance reduction half rxn
+6
Cr2O7– – → Cr3+
To balance Cr atom
Cr2O7– – → 2Cr3+
To balance O-atom
Cr2O7– – →2Cr3+ + 7H2O
To balance H-atom
Cr2O7– – +14H+ → 2Cr3+ + 7H2O
To balance charge
Cr2O7– – + 14 H+ → 2Cr3+ + 7H2O
To balance charge
Cr2O7– – + 14H+ + 6e– → 2Cr3+ + 7H2O (i)
To balance Oxidation half rxn
+4 +6
SO2 → HSO4–
To balance O-atom
SO2 + 2H2O → HSO4–
To balance H-atom
SO2+ 2H2O → HSO4– + 3H+
To balance charge
SO2 + 2H2O → HSO4– + 3H+ +2e– (ii)
Multiply eq. (2) by 3 & add both equations
3SO2 + 6H2O → 3HSO4– + 9H+ + 6e–
Cr2O7– – + 14H+ +6e– → 2Cr3+ + 7H2O
Cr2O7– – + 3SO2 + 5H+ → 2Cr3+ + 3HSO4– + H2O
f To balance the given redox-reaction
MnO4– + I– →Mn++ + I2 + H2O
+7 +2
MnO4– → Mn++ (Reduction)
0
I– →I2 (Oxidation)
To balance reduction half rxn
MnO4– → Mn++
To balance O-atom
MnO4– → Mn++ + 4H2O
To balance H-atom
MnO4– + 8H+ → Mn++ + 4H2O
To balance charge
MnO4– + 8H+ +5e– → Mn+++ 4H2O (i)
To balance oxidation half rxn
I– →I2
To balance I-atom
2I– → I2
To balance charge
2I– → I2 + 2e– (ii)
Multiply eq(1) by 2 & eq. (2) by 5 & add both equations
2MnO4– +16H++10e → 2Mn++ + 8H2O
10 I– →5I2+10e–
2MnO4– +10I– +16H+ → 2Mn++ + 5I2 + 8H2O
- g)To balance the given redox-reaction
- IO3– + Fe++ + Cl– → ICl + Fe3+
+5 +1
IO3– +Cl– → ICl (Reduction)
Fe++ → Fe3+ (Oxidation)
To balance Reduction half rxn
+5 +1
IO3– + Cl– → ICl
To balance O-atom
IO3– +Cl– → ICl + 3H2O
To balance H-atom
IO3– + Cl– + 6H+ → ICl + 3H2O
IO3– +Cl– +6H+ + 4e– → ICl + 3H2O (i)
To balance Oxidation half r×n
Fe++ →Fe3+
To balance charge
Fe++ → Fe3+ +e– (ii)
Multiply eq. (2) by 4 & add both equations
4Fe++ → 4Fe3+ + 4e–
IO3– +Cl– + 6H+ + 4e– → ICl + 3H2O
IO3– + 4Fe++ + Cl– + 6H+ → ICl+ 4Fe3+ + 3H2O
Balanced redox-reaction