Rutherford’s atomic model

source : classnotes.org.in
Rutherford’s atomic model
This atomic model is based on alpha ray scattering experiment. In this experiment a very thin foil of gold 0.0004 cm. was bombarded by alpha particles. Then most of the alpha particles pass through the foil & few are deflected from their path & very few alpha particles turned back from their original path . It means some positive charge is present in the centre. This model is also called Nuclear atomic model or Planetary model of atom.

source : NextGurukul
According to Rutherford’s atomic model,
1) An atom is very small, spherical & electrically neutral . It is made up of
i) Nucleus ii) outer region
Nucleus is present at centre of atom & is positively charged . The empty space around the nucleus is called outer region. In this region electrons are present.

source : visionlearning.com
2) All the protons & neutrons are present in the nucleus.
3) Nucleus is hard & rigid. Its radius is of the order of 10-13 – 10-12 cm.
4) In an atom number of protons is equal to the number of electrons.
5) Electrons revolve around the nucleus in a circular path.
6) Positively charged nucleus attracts the electrons of outer region but electrons do not fall into the nucleus. The reason is centrifugal force produced due to the motion of electrons is balanced by force of attraction.
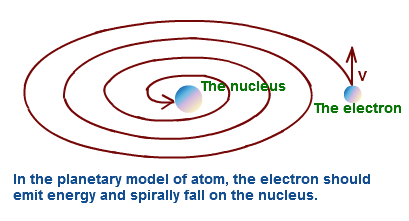
Defects of Rutherford’s atomic model-
According to this model electrons revolve around the nucleus in a circular path. A moving charged particle loses energy continuously in the form of radiation . Therefore energy of the electron decreases continuously . This will destroy the atom.But it is not possible.

source : readorrefer.in
1) Rutherford’s atomic model does not explain the stability of atom.
2) This model does not explain the line spectrum of atom.