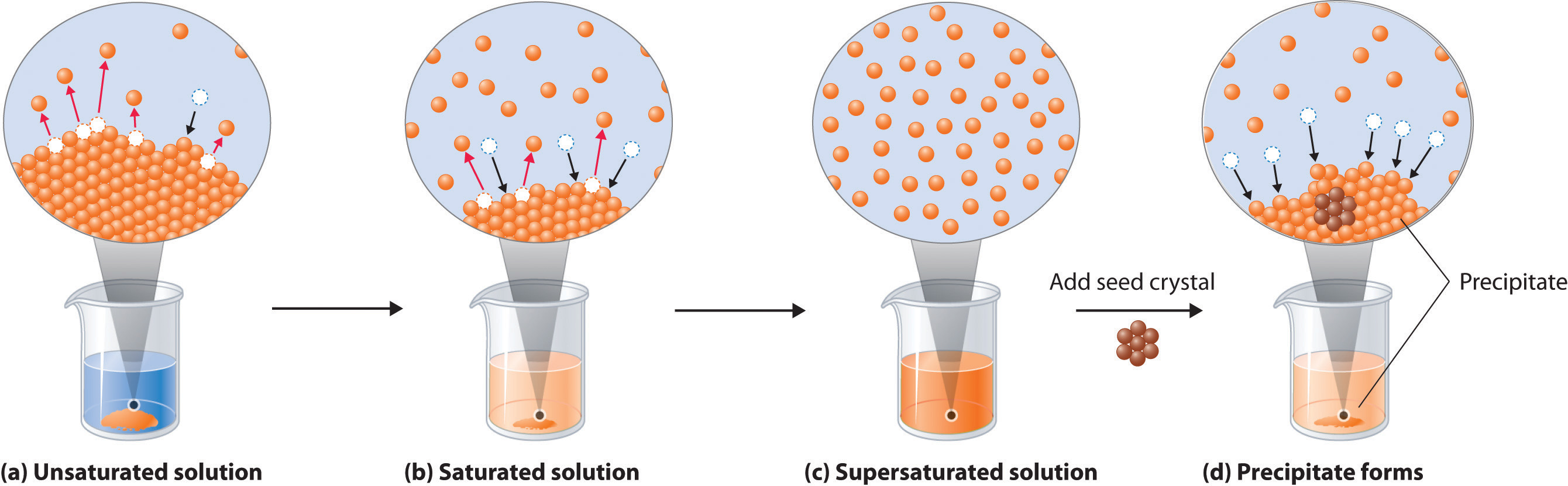
Solubility product

source : toppr
Solubility product Numerical-
Question 1) The solubility of Mg(OH)2 in a particular buffer is found to be 0.95 gm/ litre .What is the pH of the buffer solution? K sp of Mg(OH)2 = 1.8x 10-11
Solution )
S of Mg(OH)2 = 0.95 gm /litre
mol.wt. of Mg(OH)2 = 24 + (16 + 1)2 = 24 + 34 = 58
S in mole /litre = ( S in gm /litre) / mol.wt.= 0.95 / 58
S = 0.01637 = 0.0164 mole/litre
Mg(OH)2 ⇌ Mg++ + 2 OH–
Ksp = [Mg++][OH–]2
1.8 x 10-11 = 0.0164 [OH–]2
[OH–]2 = 1.8 x 10-11 / 0.0164 = 109.75 x 10-11 =10.975 x 10-10
[OH–] = √ 10.975 x 10-10 = 3.31 x 10-5 mole/litre
pOH = – log [OH–]= -log [3.31 x 10-5]
= -log 3.31 + 5 log 10= -0.5198 + 5
pOH = 4.48
pH = 14 – pOH= 14 – 4.48
pH =9.52 Ans.
Question 2) Equal volumes of 0.02 M CaCl2 and 0.0004 M Na2SO4 are mixed . Will a precipitate form ?
( Ksp of CaSO4 = 2.4 x 10-5 )
Solution )
CaCl2 + Na2SO4 ——–> CaSO4 + 2NaCl
Milli moles 0.02V 0.0004V 0 0
Assume, V ml of both are mixed.
Total volume = V + V = 2V
[Ca++] = 0.02 V/2V = 0.01
[SO4—] = 0.0004 V/2V = 0.0002 = 2 x 10-4
ionic product of [Ca++] [SO4—] =0.01 x 2 x 10-4= 2 x 10-6
K sp of CaSO4 = 2.4 x 10-5
If ionic product of [Ca++] [SO4—] is greater than K sp of CaSO4 , then precipitation takes place But,
ionic product of [Ca++] [SO4—] < K sp of CaSO4
2 x 10-6 < 2.4 x 10-5
Therefore CaSO4 will not precipitate.
Question 3) A sample of hard water contains 0.005 mole of CaCl2 per litre. What is the minimum conc. of Na2SO4 which must be added for removing Ca++ ions from this water sample? K sp of CaSO4 = 2.4 x 10-5 at 250C ?
Solution )
K sp of CaSO4 = 2.4 x 10-5
CaSO4 ⇌ Ca++ + SO4—
[SO4—] = y mole/litre is sufficient to precipitate CaSO4 from a solution having ,
[Ca++] = 0.005 mole /litre
Ksp = [Ca++] [SO4—]
2.4 x 10-5 = 0.005 y
y = 2.4 x 10-5 / 0.005= 0.48 x 10-2
y = 4.8 x 10 -3 mole/litre
Minimum conc. of Na2SO4 which must be added for removing Ca++ ions from sample = 4.8 x 10 -3 mole/litre
Question 4) The Solubility product of PbBr2 is 8 x 10-5. if the salt is 80 % dissociated in saturated solution. Find the solubility of salt in gm / litre ?
Solution )
Suppose, solubility of salt = S mole / litre
PbBr2 ⇌ Pb++ + 2 Br–
0.80 S 2 x 0.80 S
Ksp = [Pb++] [Br–]2
Solubility product of PbBr2 = 8 x 10-5
8 x 10-5 = 0.80 S ( 2 x 0.80 S)2
S3 = 3.906 x 10-5 = 39.06 x 10-6
S = 3√ 39.06 x 10-6
S = 3.39 x 10-2 mole /litre
mol.wt of PbBr2 = 207 + 2 x 80 = 367
S in gm /litre = (S in mole /litre) x mol.wt.= 3.39 x 10-2 x 367
< h4>S = 12.44 gm / litre Ans.