Structural Formula

source : source forge.net
Structural Formula
Q 1-Molecular formula of compd is C2H5NO. Compd when heated with Br2 & alc. KOH gives colourless gas of ammonical odour. Gas reacts with HCl to form white fumes . This gas on reaction with HNO2 gives alcohol & N2. Give formula of the comd &explain the reactions ?
Solution-

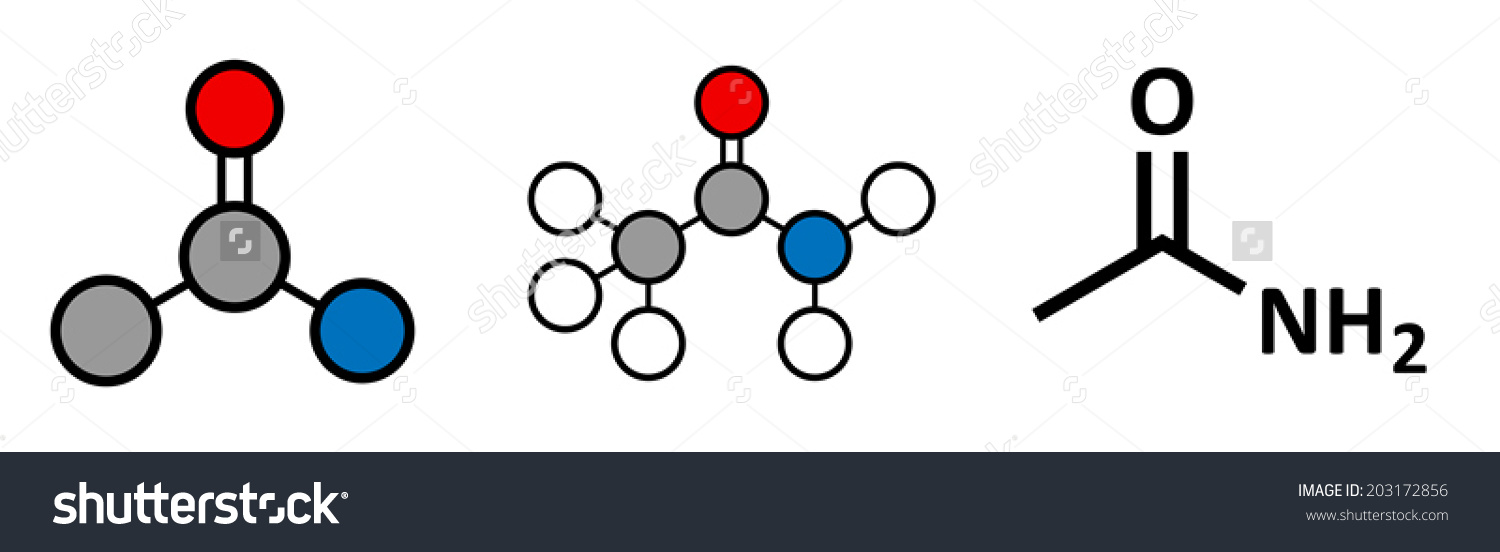
C2H5NO is an amide which is CH3CONH2
CH3CONH2 +Br2 + 4KOH(alc.)——>CH3NH2 + 2KBr +K2CO3 +2H2O
Methyl amine is colourless gas of ammonical odour , it gives white fumes with HCl.
CH3NH2 + HCl —–>CH3NH2.HCl(white fumes)
Methyl amine
CH3NH2 reacts with HNO2 gives alcohol & N2.
CH3NH2 + HNO2———>CH3OH +N2 +H2O
( alcohol )
So , compd is CH3CONH2 ( acetamide).

Q 2– Compd ‘A’ C3H6O2 on hydrolysis gives acid ‘B’ & alcohol ‘C’. Acid ‘B’ on heating with soda lime gives CH4.Give name & formula of ‘A’, ‘B’ &’C’ .
Ans. Compd ‘A’ C3H6O2 is an ester because ester on hydrolysis gives acid & alcohol. Hence ‘A’ is CH3COOCH3 (methyl acetate or methyl ethanoate).

soda lime is a mixture of NaOH & CaO.
Hence ‘A’ is CH3COOCH3 (methyl acetate or methyl ethanoate) ;’B’ is CH3COOH (acetic acid or ethanoic acid) ; ‘C’ is CH3OH (methyl alcohol or methanol).



Q 3– Molecular formula of neutral compound ‘A’ is C3H6O2. ‘A’ on hydrolysis gives monobasic acid ‘B’& a neutral compd ‘C’. Compd ‘C’ gives iodoform test . Give formula & name of ‘A’, ‘B’ & ‘C’.
Solution-
‘A’ is a neutral compd , on hydrolysis gives monobasic acid ‘B’& a neutral compd ‘C’.Therefore ‘A’ is an ester.
‘C’ gives iodoform test, hence it is C2H5OH & monobasic acid is HCOOH.
Hence ester is HCOOC2H5 (ethyl formate or ethyl methanoate).
HCOOC2H5 + H2O —->HCOOH + C2H5OH
A B C
(ethyl methanoate) ( formic acid) (ethyl alcohol)
C2H5OH +I2 + NaOH ——> CHI3 + HCOONa +NaI +H2O
iodoform
‘A’ is HCOOC2H5 (ethyl formate or ethyl methanoate) ; ‘B’ is HCOOH (formic acid or methanoic acid) ;’C’ is C2H5OH (ethyl alcohol or ethanol).



Read more articles at chemistryonline.guru