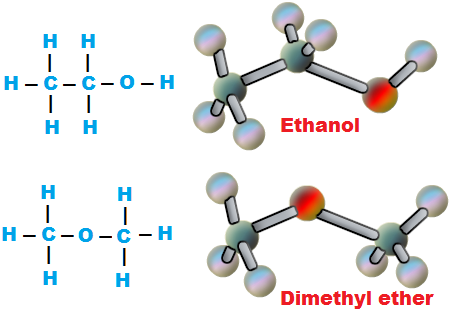
Structural Formula-
source : chem collective.org
Structural Formula-
Q 1– Two isomeric compounds ( A) & (B) have C =52.17 %; H =13.04 % & rest is oxygen.Their vapour density is 23.
Compound ‘A’ on reaction with HI gives a compound ‘C’ which when reacted with aq . NaOH gives C2H5OH . Explain the reactions.Identify A , B & C.Give their name & formula
Solution-
percentage of C= 52.17% ; % of H= 13.04 %
% of O = 100–(52.17+13.04)= 34.79 %
| Element | % | Atomic weight | Relative no. of atoms | Simplest Ratio |
| C | 52.17 | 12 | 52.17/12= 4.35 | 4.35/2.17 =2 |
| H | 13.04 | 1 | 13.04/1 = 13.04 | 13.04/2.17=6 |
| O | 34.79 | 16 | 34.79/16=2.17 | 2.17/2.17=1 |
Empirical formula =C2H6O
mol.formula=(C2H6O)n
Empirical formula weight =12 x 2+6 x 1+16 =46
mol. wt. = 2 x V.D =2 x 23=46
n = mol.wt. /Empirical formula weight
=46/46=1
n =1
mol.formula = (C2H6O)n
mol.formula = (C2H6O)1 =C2H6O
The possible isomers of C2H6O are, C2H5OH & CH3OCH3

Compound A is ethanol because it on reaction with HI gives’ C’ i.e C2H5I . Compd ‘C’ on hydrolysis with aq.NaOH gives ethyl alcohol.


C2H5 I + NaOH(aq.)—–>C2H5OH +NaI
compound A is C2H5OH(Ethyl alcohol) ; compound B is CH3OCH3 (dimethyl ether) ; compound C is C2H5I(Ethyl iodide).



Q 2– Molecular formula of compd ‘A’ & ‘B’ is CH2O & C2H4O2 respectively . ‘A’ reduces ammonical AgNO3 solution but ‘B’ does not.The neutral solution of ‘B’ gives a red colour with neutral FeCl3.Find the formula of both compds & give their names?
Ans.
Molecular formula of A is CH2O.
Because it reduces ammonical AgNO3 solution hence it is an aldehyde. Thus compd is HCHO.
So ‘A’ is HCHO ( formaldehyde).
Molecular formula of ‘B’ is C2H4O2.
Hence ‘B’ gives red colour with neutral FeCl3 hence it is an acid . Thus compd is CH3COOH.
So ‘B’ is CH3COOH ( acetic acid).


Q 3– Molecular formula of compd ‘A’ & ‘B’ is CH2O & C2H4O2 respectively . ‘A’ reduces fehling solution but does not react with Na2CO3 solution. ‘B’ does not reduce fehling solution but gives effervesence with Na2CO3.Find the name & formula of both compds?
Ans.
Molecular formula of A is CH2O.
Because it reduces fehling solution hence it is an aldehyde. Thus compd is HCHO.
So ‘A’ is HCHO ( formaldehyde).
Molecular formula of ‘B’ is C2H4O2.
As we know that ‘B’ gives effervesence with Na2CO3. An acid gives effervesence with Na2CO3 solution. hence it is an acid . Thus compd is CH3COOH.
So ‘B’ is CH3COOH ( acetic acid).


Read more articles at chemistryonline.guru