Optical Activity-

source: en.wikipedia.org
Optical Activity-
“Substances which rotate the plane of plane polarized light are known as optically active and the phenomenon is called optical activity.
Plane polarized light-
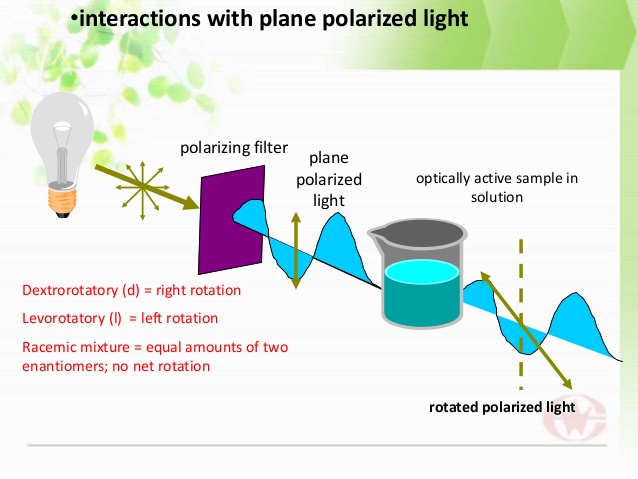
when ordinary light is passed through a nicol prism, then all these vibrations are cut off leaving behind vibrations in only one plane.This vibration of one plane is called plane polarized light.

when plane polarized light is passed through solution of certain substances then plane of plane polarized light is rotated either right (clockwise) or left (anti-clock wise).
The substances which rotates the plane of plane polarized light to right,then substance is dextro-rotatory. Therefore it is indicated by (+) sign.

If it rotates the plane of plane polarized light to left, then substance is laevo-rotatory.Thus it is indicated by (- ) sign.

The magnitude of rotation depends upon following factors:-
- Concentration of the solution.
- length of solution in polarimeter tube.
- wave length of light.
- Nature of solvent.
- Nature of compound.
-
Temperature of solution.
Necessary Conditions For Optical Activity-
Compound which is super imposable on its mirror image , show optical activity. It may be due to,
(1)- Its assymetric crystalline structure
(2)- assymetry in its molecular structure
Optical activity due to asymmetry of crystalline structure-
The quartz crystal is optically active in solid state only, the two forms are mirror images of each other but are not super imposable images.But in fused state its optical activity is destroyed.
Optical activity due to asymmetry of molecular structure-
The Compounds having one or more asymmetric carbon atom show optical activity. But some compounds do not have asymmetric carbon atom even then they do not show this activity. Hence the necessary condition for a molecule to show this phenomenon is asymmetry of molecule as a whole. A molecule is asymmetric if it does not have any element of symmetry.
Element of symmetry- A molecule may have any of symmetries :
- Plane of symmetry.
- Center of symmetry.
- An alternating axis of symmetry.
Plane of symmetry: It is an imaginary plane that divides a molecule into two halves, which are mirror images of each other.


Centre of symmetry: It is an imaginary point with in a molecule from which lines when drawn on , one side & extended the opposite side by equal distance , will meet the identical atoms or groups in the molecul


Alternating axis of symmetry: if the molecule is rotated through an angle of 360/n about this axis then molecule possess ‘n’ fold alternating axis of symmetry .
Optical activity is due to molecular asymmetry of compound.Such compounds have one or more asymmetric carbon atom (such as lactic acid ) & its mirror image is non super imposable. So it is optically active.


Note : some compounds (like biphenyl, allenes) have no asymmetric C-atom but their images are non super imposable because of molecular asymmetry and these compounds are optically active.
read more articles at chemistryonline.guru