Third order reaction-

source : SlideShare
Third order reaction-
” A reaction is third order if rate of reaction is determined by variation of three concentration terms.”
Ex –
1) 2NO +O2 ——–> 2NO2
2) 2NO + Cl2 ———> 2NOCl
3) 2FeCl3 + SnCl2 ——–> 2FeCl2 + SnCl4
consider a reaction,
A+B+C ———> product
Rate of reaction,
dx/dt = K (a- x )(b- x )(c- x )
When ,
a = b = c , then
dx/dt ∝ (a- x )3
dx / (a – x )3 = K.dt
Taking integration of both sides,
∫ dx / (a – x )3 = K∫ dt
1 / 2 (a – x)2 = Kt + C ——— eq.1
when t = 0 then x = 0
1 / 2 ( a – 0)2 = K x 0 + C
C = 1/ 2a2
Putting the value of ‘C’ in eq. 1
1 / 2(a – x)2 = Kt + 1 / 2a2
K = 1/ 2t [ (1 / (a- x)2 – (1/a2)]
K = 1 / 2t {a2-(a2 +x2-2ax)}/ {a2(a-x)2}]
K = 1/2t [ ( 2ax -x2 ) /{a2(a-x)2}
K = 1/2t [ {x(2a- x) }/{a2(a-x)2}
a = initial concentration
a – x = conc. of reactant after time ‘t’
Characteristics of third order reaction –
1) Unit of ‘K’-
K = 1 / 2t [x( 2a-x)/ a 2(a – x)2]
unit of ‘K’ = 1 / time ( mole litre-1 ) (mole litre-1)/( mole litre-1 )2 x (mole litre-1)2
unit of ‘K’ = litre2 mole -2 time-2
2) The unit of velocity constant depends upon the units of concentration because,
unit of ‘K’ = litre 2mole -2 time-2
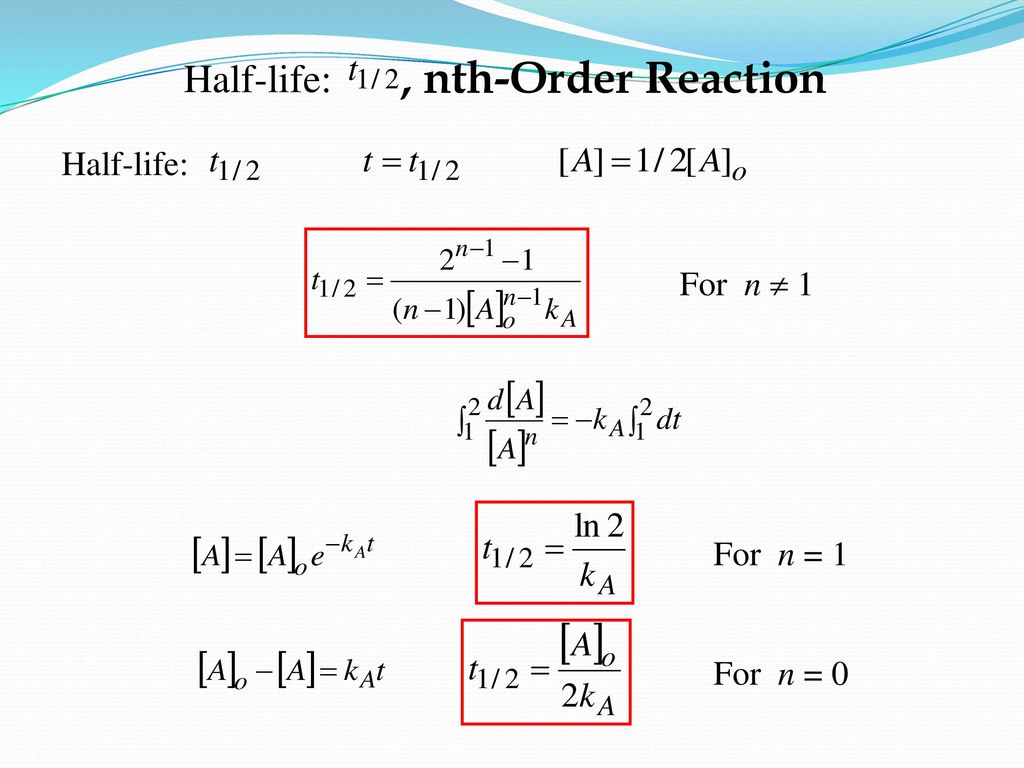
3) The time taken to complete a half reaction( half life period ) is inversely proportional to the square of initial concentration of the reactant.
K = 1 / 2t [x( 2a-x)/ a 2(a – x)2]
If t = t1/2 then x = a/2
K = 1/ 2t1/2 [ {a/2} {2a- (a/2)}] / [ 2a2(a -a/2)2]
t 1/2 = 1 / 2K [ (a/2) (3a/2)]/ [2a2( a2/4)]
t 1/2 = 3/ 2Ka2
t 1/2 ∝ 1 /a2