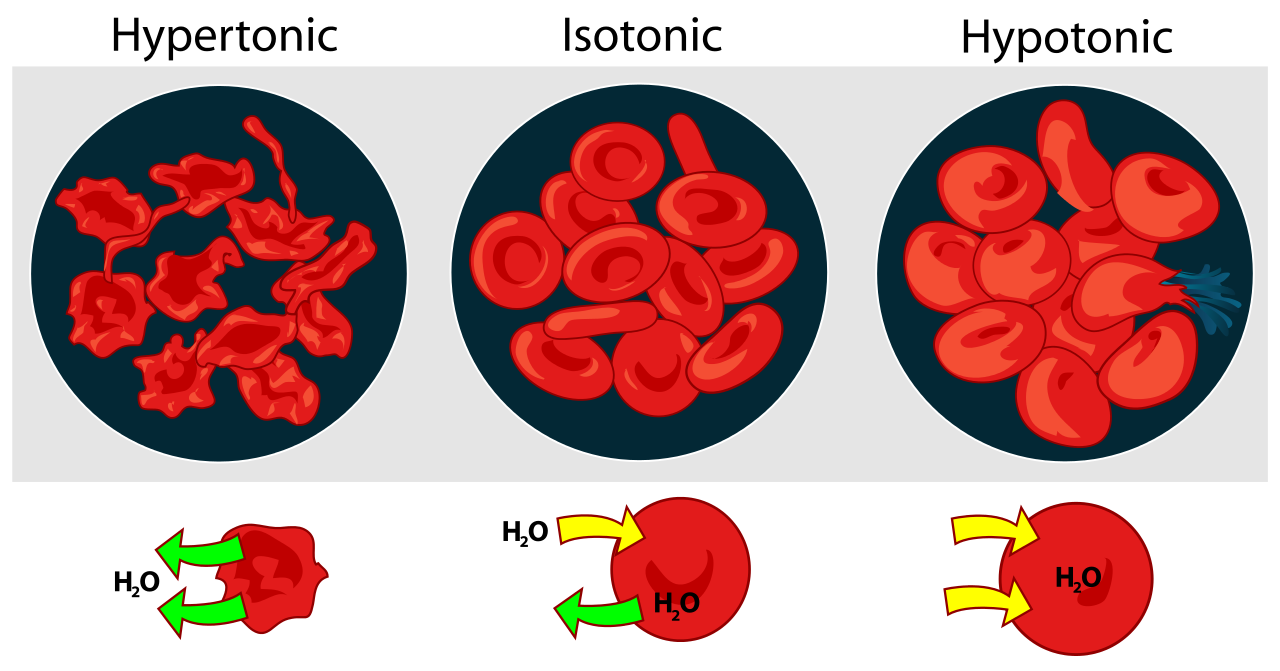
osmotic pressure

source : SlidePlayer
osmotic pressure-
Question 1) At 298 K , 100 cc of a solution containing 3.02 gm. of a solute exhibits an atmospheric pressure of 2.55 atmosphere. What is the molecular mass of the solute ?
Solution )
w = 3.02 gm , V = 100 cc = [100/1000] litre = 0.1 litre
π = 2.55 atm , m = ? , S = 0.0821 Litre atm K-1mole-1 , T = 298 K
π = w ST /m V
m = w S T /π V = 3.02 x 0.0821 x 298 /2.55 x 0.1
m = 289.75 Ans.
Question 2) 0.85 % aqueous solution of NaNO3 is apparently 90 % dissociated at 270 C . Calculate osmotic pressure of the solution ?
(S = 0.0821 Litre atm K-1mole-1 )
Solution )
w = 0.85 gm , V = 100 ml = [100/1000] litre = 0.1 litre
π = ? atm , m of NaNO3 = 23 + 14 +16 x 3 = 85 , S = 0.0821 Litre atm K-1mole-1 , T = 273 + 27 =300 K
π N = w ST /m V
π N = 0.85 x 0.0821 x 300 / 85 x 0.1
π N= 2.463 atm. Ans.
NaNO3 ——–> Na + + NO3–
mole before dissociation 1 0 0
moles after dissociation 1 – ∝ ∝ ∝
Total moles after dissociation = 1 – ∝ + ∝ + ∝ = 1 + ∝
% of ∝ = 90 % , ∝ = 90 / 100 = 0.90
π exp / π N = (1 + ∝ ) / 1
π exp / 2.463 = ( 1 + 0.90 )
π exp =1.90 x 2.463
π exp = 4.679 atm Ans.

source : Biology Stack Exchange
Question 3 ) Calculate the osmotic pressure of 20 % ( weight / volume ) anhy. CaCl2 solution at 0 0 C ? . Assuming 100 % ionisation.
Solution )
w = 20 gm , V = 100 ml = [100/1000] litre = 0.1 litre
π = ? atm , m of CaCl2 = 40 + 71 = 111 , S = 0.0821 Litre atm K-1mole -1, T = 0 + 273 = 273 K
π N = w ST /m V
π N = 20 x 0.0821 x 273 / 111 x 0.1
π N = 40.38 atm. Ans.
CaCl2 ——–> Ca++ + 2 Cl-1
mole before dissociation 1 0 0
moles after dissociation 1 – ∝ ∝ 2 ∝
Total moles after dissociation = 1 – ∝ + ∝ + 2∝ = 1 + 2∝
% of ∝ = 100 % , ∝ = 100 / 100 = 1.0
π exp /π N = (1 + 2∝ ) / 1
π exp / 40.38 = ( 1 +2 x 1)
π exp = 3 x 40.38
π exp = 121.14 atm Ans.
Question 4) The average osmotic pressure of human blood is 7.7 atm. at 40 0 C. Calculate assuming molarity & molality be same.
a) concentration of blood in molarity
b) Freezing point of blood
Given Kf of H2 O is 1.86
Solution )
a) π = 7.7 atm , T = 40 + 273 = 313 K
π = CST
C = π /ST=7.7 /0.0821 x 313
C = 0.299 = 0.3 M Ans.
b)
ΔTf = Kf x molality
Kf = 1.86
molality = 0.3
ΔTf = 1.86 x 0.3=0.558
Depression in freezing point (ΔTf ) = freezing point of solvent – freezing point of solution( blood)
0.558= 0 – freezing point of solution(blood)
freezing point of solution(blood) = 0 – 0.558
freezing point of solution (blood) = – 0.558 0C Ans.