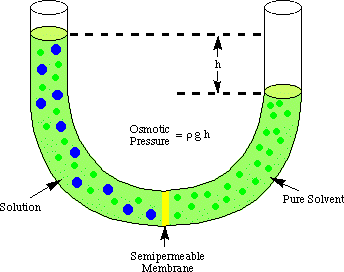
Osmotic pressure-

source : hindi.theindianwire.com
Osmotic pressure-
Q 1) A decimolar solution of potassium ferrocyanide is 50% dissociated at 300k. Calculate osmotic pressure of the solution ?
S = 8.314 JK-1 mole-1
Solution )
molarity of solution = decimolar means 0.1 mole/litre = 1/10 mole /litre = no. of moles / volume of solution (litre)
π = ? atm , S = 8.314 J K-1mole-1 , T =300 K ,n / v = 0.1 mole / litre = [0.1 / 10 -3 ]mole /m3 = 100 mole/m3
π N = n ST / V
π N = 100 x 8.314 x 300
π N= 2.49 x 105 N/m2
K4 [Fe (CN)6] ——–> 4 K + + [Fe (CN)6] 4-
mole before dissociation 1 0 0
moles after dissociation 1 – ∝ 4 ∝ ∝
Total moles after dissociation = 1 – ∝ + 4∝ + ∝ = 1 + 4∝
% of ∝ = 50 % , ∝ = 50 / 100 = 0.50
π exp / π N = (1 + 4∝ ) / 1
π exp / 2.49 x 105 = ( 1 + 4 x 0.50 )
π exp = 2.49 x 105 x 3
π exp = 7.47 x 105 N/m2 Ans.
Q 2) 7.6 gm KBr in 1250 ml solution was found to have an osmotic pressure of 1.804 atm at 270 C . Calculate degree of ionisation & vant Hoff factor (i)?
Solution )
π exp = 1.804 atm.
w = 7.6 gm , V = 1250 ml = 1250 / 1000 = 1.25 litre
π = ? atm , m of KBr = 39 + 80 = 119 , S = 0.0821 Litre atm K-1mole-1 , T = 273 + 27 =300 K
π N = w ST /m V
π N = 7.6 x 0.0821 x 300 / 119 x 1.25
π N= 1.258 atm. Ans.
KBr ——–> K+ + Br –
mole before dissociation 1 0 0
moles after dissociation 1 – ∝ ∝ ∝
Total moles after dissociation = 1 – ∝ + ∝ + ∝ = 1 + ∝
π exp / π N = (1 + ∝ ) / 1
1.804 / 1.258 = ( 1 +∝ )
1.804 =1.258 (1 + ∝)
1.804 = 1.258 + 1.258 ∝ Ans.
1.258 ∝ = 1.804 – 1.258
1.258 ∝ = 0.546
∝ = 0.546/ 1.258
∝ = 0.434
% of ∝ = 0.434 x 100 = 43.4 % Ans.
i = no. of particles after dissociation / no. of particles before dissociation
i = ( 1 +∝ ) / 1
i = 1 + 0.434
i = 1.434 Ans.
Alternative method for Vant Hoff factor –
i= π exp /π N
i = 1.804 / 1.258
i = 1.434 Ans.
Q3) Osmotic pressure of a solution containing 7 gm of a dissolved protein in 100 ml of solution is 20 mm of Hg at 370 C. Find the molecular weight of protein ?
Solution )
w = 7.0 gm , V = 100 ml = 100 / 1000 = 0.1 litre
π = 20 mm = 20 /760 atm , m of protein = ? , S = 0.0821 Litre atm K-1mole-1 , T = 273 + 37 =310 K
π = w ST /m V
m = wST /π V
m = 7.0 x 0.0821 x 310 x 760 / 20 x 0.1
m = 67699.66 atm. Ans.
Question 4 ) Calculate the osmotic pressure of a solution having 3.42 gm /dm3 sucrose at 270C?
Solution )
w / V = 3.42 gm / dm3 = [3.42 x 10 -3 /10 -3 ]Kg / m3 ,
π = ? N / m2 , m of sucrose [C12H22O11] = 342 , S = 8.314 J K-1mole-1 , T = 273 + 27 =300 K
π = w ST /m V
π = 3.42 x 10 -3 x 8.314 x 300 / 342 x 10 -3
π = 24.95 N/m2 Ans.