Active mass

source : chem4kids.com
Active Mass-
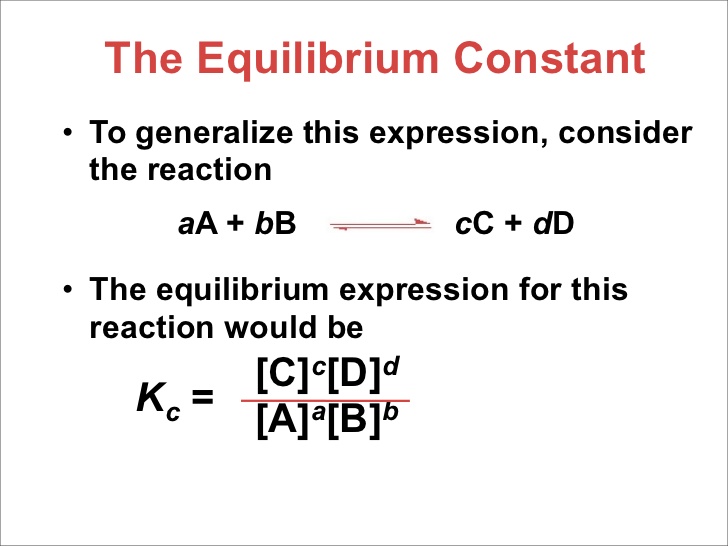
Active mass of a substance is its molar concentration or molarity. Number of moles present in one litre of solution is active mass of that substance. Its unit is mole /litre. Active mass of a substance is represented by enclosing its formula in square brackets
Active Mass of Substance = number of moles /volume of solution in litre
No. of Moles = w/m =weight of solute /molecular weight
Active Mass = w /mV(l)
Q.1 In the pot of capacity 2 litre at 4000C, 4 gm of H2 & 128 gm of HI was taken. Calculate the active mass or molar concentration ?
Ans. w of H2 =4 gm.
V = 2 litre
m of H2 = 2
[H2] =w/mV(l)
=4 /2 x2 =1 mole /litre Ans.
[HI] =w/mV
w of HI =128 gm.
m of HI =128
=128/128 x 2 =0.5 mole /litre Ans.
Q 2—In 500 ml of a Soln 15 gm acetic Acid & 8 gm. Methanol is present (CH3OH) what will be their active masses?
Ans V=500ml ==0.5 l
w of CH3COOH= 15gm
mol.wt. of CH3COOH= 60
[CH3COOH] = w/mV
=15 /60 x 0.5 =0.5 mole /litre Ans.
w of CH3OH=8gm
m of CH3OH=32
[CH3OH] = w/mV
=8 /32 x 0.5 =0.5 mole /litre Ans.
Q 3—In 1 litre gaseous mix. 1 gm H2 & 2.8 gm N2 is present. Calculate the molar concentrations ?
Ans. w of H2= 1gm
w of N2 = 2.8 gm
v= 1 litre
m of H2=2
m of N2 =28
[H2] = w/mV
=1 /2 x 1 =0.5 mole /litre
[N2] = w/mV
=2.8 /28 x 1 =0.1 mole /litre
[H2] =0.5 mole/litre; [N2] =0.1 mole /litre
Q 4—4 litre of a gaseous mix. contains 4 gm. H2, 142 gm. Cl2 & 73 gm. HCl. Calculate their active masses?
V=4 litre
w of H2 = 4 gm, m of H2 = 2
[H2] = w/mV
4 /2 x 4 =0.5 mole/litre
w of Cl2 = 142 gm, m of Cl2 = 71
[Cl2] = w/mV
=142 /71 x 4 =0.5 mole /litre
w of HCl = 73 gm, m of HCl = 36
[HCl] = w/mV
=73 /36.5 x 4 =0.5 mole /litre Ans.
Q 5—In the pot of capacity of 2 l, 64 gm. of HI is present. Calculate its active mass?
Ans. V=2 l, w of HI = 64 gm. m of HI= 128
[HI] = w/mV
=64 /128 x 2 =0.25 mole /litre
Q 6—In 500ml of solution 46 gm of C2H5OH & 15 gm. of Acetic Acid are present. Calculate their active masses.
Ans. V =500 ml= 0.5 l
w of C2H5OH= 46
m of C2H5OH =46
[C2H5OH] = w/mV
=46 /46 x 0.5 =2 mole /litre
w of CH3COOH= 15 gm
m of CH3COOH=60
[CH3COOH] = w/mV
=15 /60 x 0.5 =0.5 mole /litre Ans.