TYNDALL EFFECT & BROWNIAN MOVEMENT

source : buzzle.com
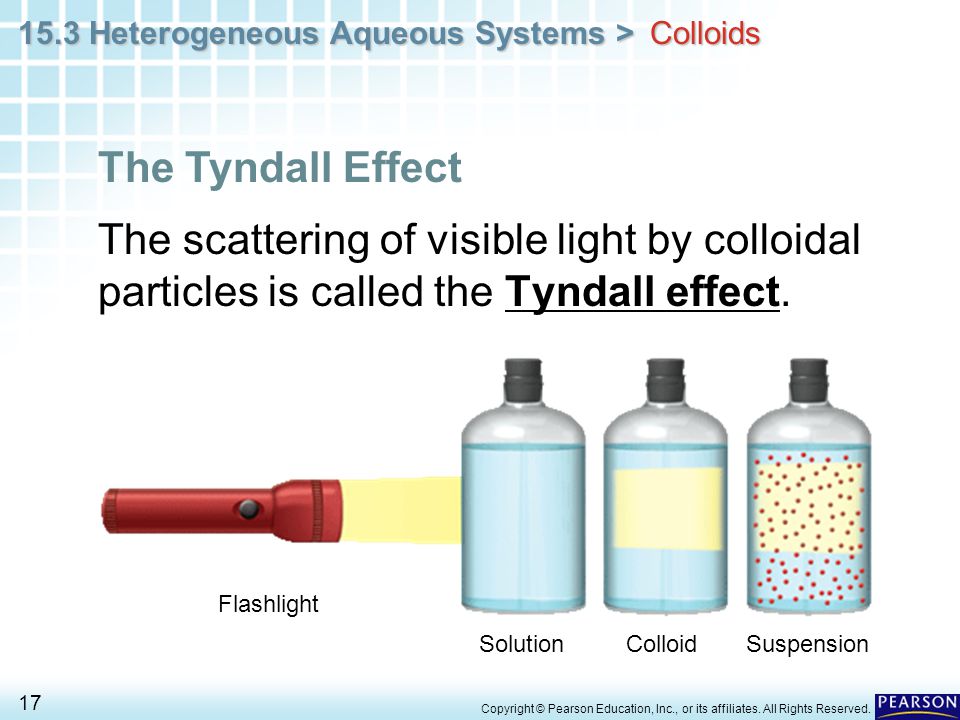
TYNDALL EFFECT
Tyndall effect is an optical property of colloidal solution. When a convergent beam of light is passed through a colloidal solution. It is scattered, the maximum scattered intensity being in the plane at right angle to the path of light. Thus the path of the light is also visible as two cones. This optical phenomenon was first observed by Faraday but was studied in detail by Tyndall, hence the effect is known as Tyndall effect.
The phenomenon of scattering of light by the colloidal particles due to which the path of beam is illuminated by a bluish light when seen at right angle to the path of light, is called Tyndall effect.”
The two bright cones indicating the path of light are known as Tyndall cones.
source : ask iitians.co
Reason of Tyndall effect:
The size of colloidal particles are smaller than wave length of light, therefore colloidal particles can not reflect light but scattering of light take place. Hence tyndall effect is due to scattering of light by colloidal particles.
Brownian Movement-
It is a kinetic property. When colloidal solution are viewed under powerful ultra microscope, then colloidal particles are moving rapidly is zig-zag directions. This motion was first observed by British Botanist Robert Brown & hence is known as Brownian movement. (Note-True solution & suspension do not show this movement.)
This motion can be observed by viewing dust particles in the path of light coming into a dark room through a small hole. Dust particles are clearly seen as bright spots moving randomly in zig-zag direction.

source : spm chemistry.onlinetution.com.my
Reason
The Brownian movement of particles is due to the unbalanced bombardment of the particles by the molecules of dispersion medium.
Read more articles at chemistryonline.guru