Aufbau principle
 source : slide player.com
source : slide player.com
Aufbau (German word) means ‘building up’ according to Aufbau principle,
“The vacant sub shell having lowest energy is filled first. When this sub shell is filled completely, then the filling of next sub-shell with higher energy starts.”
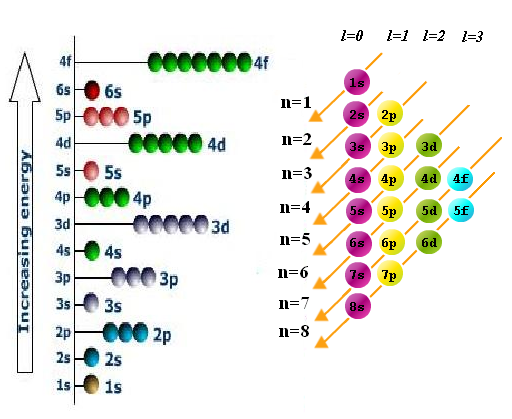
The energy level diagram is,

Order of Filling of Subshell
According to Aufbau principle energy of various sub shell increases in the order.
1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d<7p
| 1H -1s1
2He – 1s2 3Li – 1s2, 2s1 4Be -1s2, 2s2 5B – 1s2, 2s2,2p1 6C – 1s2,2s2,2p2 7N – 1s2,2s2,2p3 8O -1s2,2s2, 2p4 9F -1s2,2s2,2p5 10Ne -1s2, 2s2,2p6 11Na -1s2 , 2s2, 2p6, 3s1 12Mg -1s2, 2s2, 2p6, 3s2 13Al -1s2 , 2s2, 2p6, 3s2, 3p1 14Si – 1s2 ,2s2, 2p6, 3s2, 3p2 15P – 1s2 , 2s2, 2p6, 3s2, 3p3
|
16S 1s2 , 2s2, 2p6, 3s2, 3p4
17Cl 1s2 , 2s2, 2p6, 3s2, 3p5 18Ar 1s2 , 2s2, 2p6, 3s2, 3p6 19K 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s1 20Ca 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s2 21Sc 1s2 , 2s2 , 2p6 , 3s2, 3p6, 4s2, 3d1 22Ti 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s2, 3d2 23V 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s2, 3d3 24Cr 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s1, 3d5 25Mn 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s2, 3d5 26Fe 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s2, 3p6 27Co 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s2, 3d7 28Ni 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s2, 3d8 29Cu 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s1, 3d10 30Zn 1s2 , 2s2 , 2p6, 3s2, 3p6, 4s2, 3d10 |
There are few exceptions to Aufbau principle , such as
24Cr 1s2 , 2s2 , 2p6 , 3s2, 3p6, 4s1, 3d5
29Cu 1s2 , 2s2 , 2p6 , 3s2, 3p6, 4s1, 3d10
41Nb 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10, 4p6 , 5s1, 4d4
42Mo 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10, 4p6, 5s1, 4d5
44Ru 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10, 4p6, 5s1, 4d7
45Rh 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2, 3d10, 4p6, 5s1, 4d8
46Pd 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10, 4p6, 5s0, 4d10
47Ag 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10, 4p6, 5s1, 4d10
78pt 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10 , 4p6 , 5s2 , 4d10 , 5p6, 6s0, 4f14, 5d10
79Au 1s2 , 2s2 , 2p6 , 3s2 , 3p6, 4s2, 3d10, 4p6 , 5s2 , 4d10 , 5p6, 6s1, 4f14, 5d10
Give electronic configuration of given ions according to Aufbau principle
11Na+, 12Mg++, 9F–, 8O––, 29Cu++, 29Cu+, 24Cr+, 24Cr3+, 25Mn++, 22Ti++, 30Zn++
No of electrons in Na+=11-1=10
Na+=1s2,2s2, 2p6
no.of electrons in Mg++=12-2=10
Mg++=1s2,2s2,2p6
no. of electrons in F–= 9+1=10
F–=1s2,2s2,2p6
no. of electrons in O– –= 8+2=10
O– –=1s2,2s2,2p6
no. of electrons in Cu+=29-1=28
Note : When d- sub shell is present then electrons are removed from outer most shell (n), then penultimate shell (n–1)d.
Cu 1s2, 2s2 , 2p6, 3s2, 3p6, 4s1, 3d10
Cu+ 1s2, 2s2, 2p6, 3s2, 3p6, 4s0, 3d10
Cu++ 1s2, 2s2, 2p6, 3s2, 3p6, 4s0, 3d9
24Cr 1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d5
Cr+ 1s2, 2s2, 2p6, 3s2, 3p6, 4s0, 3d5
Cr3+ 1s2, 2s2, 2p6, 3s2, 3p6, 4s0, 3d3
25Mn 1s2, 2s2, 2p6, 3s2, 4s2, 3d5
Mn++ 1s2, 2s2, 2p6, 3s2, 3p6, 4s0, 3d5
22Ti 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d2
Ti++ 1s2, 2s2, 2p6 , 3s2, 3p6, 4s0, 3d2
30Zn 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10
Zn++ 1s2, 2s2, 2p6, 3s2, 3p6, 4s0, 3d10