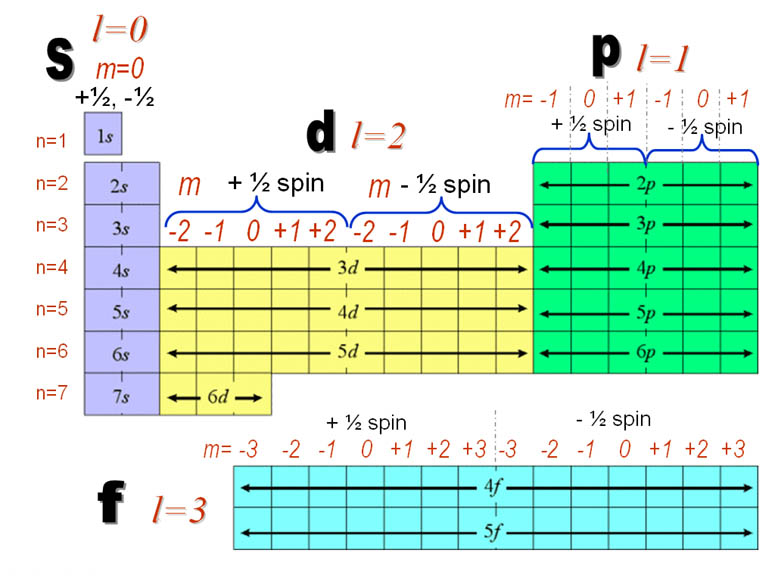
Quantum number

source : physics.tutor vista.com
Quantum numbers
Question1: What is value of Principal & Azimuthal Quantum Number of e’s of 3d subshell.
Ans. n=3, l=2
Question2. What is the subshell of electron with quantum numbers-
n=4, l=3, m=0, s=-1/2
Ans. 4f
Question3.Which set the quantum number is not possible?
n l m s
(a) 3 2 -2 +1/2
(b) 4 0 0 -1/2
(c) 3 2 -3 +1/2
(d) 5 3 0 -1/2
Ans. (c) is not possible because if l=2 then values of m= -2,-1,0,+1, +2
Question4 . Which set the quantum number is not possible?
- a) n=1, l=1, m=1, s=+1/2
- b) n=1, l=0 m=1, s=+1/2
- c) n=1, l=0, m=0, s=-1/2
- d) n=2, l=0 m=0 s=+1/2
Ans. (a)& (b) are not possible because, n=1, then l=0 to (n-1)
so if n=1, then l=0
if l=0, then m= -l to +l including zero, if l=0, m ≠ 1
Question 5. What is the maximum No. of Electrons present in the subshell for n=3,l=1.
Ans. if n=3, l=1, then subshell is 3p.
p- subshell accommodate 6 electrons
Question. 6. How many orbitals are possible for n=3 ?
Ans. If n=3, l=0,1,2 means s,p,d
s- subshell= One s-orbital
p-subshell = Three p-orbital
d-subshell = Five d-orbital
Thus for n = 3 , the possible no. of orbitals = 1+3+5 = 9
The possible no. of orbitals = 9
Question7. Write the possible values of l, m & s for the Principal Q. No. n=1
Ans. If n=1 then values of l=0 to (n-1)
l=0
for l=0, m=0
for m=0, values of s=+1/2 & -1/2
n=1, l=0, m=0, s=+1/2 & -1/2
Question 8 . Write the values of l & m for n=2
Ans. If n=2, l=0 to (n-1)
Hence , l = 0,1
Values of m= -l to +l including zero.
for l=0, m=0
l=1, m=-1,0,+1
Question 9. Write the value of m for l=3
Ans. Values of m= – l to +l including zero
m= -3, -2,-1, 0,+1, +2,+3
Question 10 . Write the values of l & m for n= 3
Ans. n=3, l=0,1,2
If l=0, m=0
l=1, m=-1,0,+1
l=2, m=-2,-1,0,+1,+2
Question 11. Write all the possible values of l, m & s for n=3
Ans. n =3 , l=0,1,2
If l= 0 (s-subshell) , Value of m= 0 , value of s = ±1/2
If l=1 (p-subshell) , Value of m = -1 ,0,+1 , value of s = ±1/2 for each value of m
If l = 2(d-subshell) , Value of m = -2 ,-1,0,+1,+2 , value of s= ±1/2 for each value of m
Question 12. Calculate maximum Number of e’s in n=4
Total electrons = 2+6+10+14=32
Question 13. What information does 3d5 give about Q. No.?
Ans. for 3d5
n=3, l=2 and five electrons are present in d-subshell
Question 14.What information does 2s2 give about Q. No.?
Ans. for 2s2
n=2, l=0 and two electrons are present in s-subshell
Question15 .Write the values of n & l for the following subshell
a) 3s (b) 5p (c) 4d, (d) 4f
Ans. (a) for 3s: n=3, l=0
(b) for 5p : n=5, l=1
(c) for 4d : n=4, l=2
(d) for 4f : n=4, l=3
Question16.Write the values of four Q. Numbers for all the 2s2 electrons
Ans. For 2s electrons:
for First e n=2, l=0, m=0, s=+1/2
for second e n=2, l=0, m=0, s=-1/2
Question 17.Write the values of four Q. Nos. for all the 3p6 electrons
Ans.
Electrons 1,4 2,5 3,6
for First e : n=3, l=1, m=-1, s=+1/2
for Second e : n=3, l=1, m=0, s=+1/2
for Third e : n=3, l=1, m=+1, s=+1/2
for Fourth : n=3, l=1, m=-1, s=-1/2
for fifth e : n=3, l=1, m=0, s=-1/2
for sixth e : n=3, l=1, m=+1, s=-1/2
Question 18. Write the Q. Nos. for the electron of highest energy in calcium & sodium.
Ans. 20Ca : 1s2, 2s2, 2p6, 3s2, 3p6, 4s2
Highest energy electron in Ca is 4s2
n= 4, l= 0, m= 0, s= -1/2
11Na : 1s2, 2s2, 2p6, 3s1
Highest Energy electron in Na is 3s1
n= 3, l= 0, m= 0, s = +1/2
Question 19.Write all the four Q. Nos. of 5th electron of 3d10
Ans.
n=3, l=2, m=+2, s=+1/2
Question 20 . Write all the four Q. Nos. of 10th & 20th electron of Ca .
Ans. 20Ca– 1s2, 2s2, 2p6, 3s2, 3p6, 4s2
10th electron is last electron of 2p6
n= 2, l= 1, m= +1, s= -1/2
20th electron is 4s2 ,
n=4, l=0, m=0, s=-1/2
Question 21. Write all the four Q. Nos. of all the electrons present in the d-orbitals of 24Cr.
Ans. 1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d5
for 3d1 electron : n= 3, l= 2, m= -2, s = +1/2
for 3d2 electron : n= 3, l= 2, m= -1, s = +1/2
for 3d3 electron : n= 3, l = 2, m= 0, s = +1/2
for 3d4 electron : n= 3, l = 2, m = +1, s=+1/2
for 3d5 electron : n= 3, l = 2, m = +2, s=+1/2
Question 22. How are the following subshell represented?
a) n =2, l= 1, b) n = 4, l = 3, c) n=3, l=2 d) n= 5, l= 0
Ans.
a) n=2 Second shell b) n=4, Fourth shell
l = 1 p-subshell l =3 means f-subshell
subshell = 2p subshell = 4f
c) n= 3, l= 2 d) n= 5, l= 0
l=2 means d-subshell l= 0 means s-subshell
subshell = 3d subshell = 5s