Carbenes

source : Quora.com
Carbene
These are highly reactive neutral species containing a divalent carbon. It acts as reaction intermediate.In carbenes , carbon atom has four electrons in the valence shell of which two electrons are unshared.
Ex. – :CH2 (methylene carbene) , :CCl2 (dichlorocarbene)
:CH2 (methylene carbene) is parent carbene from which all other carbene compounds are derived.
Methods of preparation-
1) By thermal decomposition of diazo methane –
Methylene carbene is formed
CH2N2 ——— > :CH2 + N2
2) From halogen –
Carbene is formed by the alpha elimination of HX from haloform with base.
CHCl3 + B– ————> BH + :CCl2 (dichloro Carbene) + Cl–
3) By photochemical decomposition of ketene-
Carbene is formed.
CH2CO ———> :CH2 (Carbene) + CO
Structure –
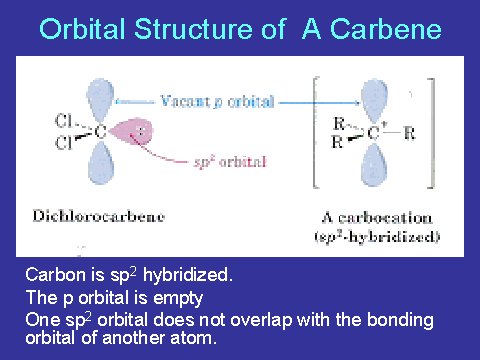
Carbenes are classified as either SINGLET CARBENE & TRIPLET CARBENE.
SINGLET CARBENE-
In singlet state carbon atom is in sp2 hybridisation state. One of sp2 hybrid orbital contains unshared electron pair with opposite spins & two sp2 hybrid orbitals form two covalent bonds . The geometry is planar.There is no magnetic moment in this state.

source : Quora
TRIPLET CARBENE –
In many triplet Carbene , carbon atom is in sp hybridization state. Two hybrid orbitals form sigma bond . Two remaining electrons with parallel spin occupy mutually perpendicular py & Pz orbitals. Geometry is linear . Such carbene would have magnetic moment.

Triplet carbenes are also known where carbon atom is in sp2 hybridisation state.Two nonbonding electrons occupy an sp2 atomic orbital & an unhybridized p-atomic orbital with parallel spin.Their geometry is bent .

source : OChemPal
Applications-
Carbene acts as reaction intermediate in so many organic reactions .
1) Carbyl amine reaction –
DichloroCarbene is reaction intermediate in this reaction.
RNH2 + CHCl3 + KOH (alc. ) ———–> RNC + 3KCl + 3 H2O
2) Reimer Tiemann reaction –
DichloroCarbene is reaction intermediate in this reaction .
3) Wittig reaction –
![]()
4) In the preparation of allenes-

Stability of Carbenes –
Carbenes in which the carbon of carbene is attached to two atoms , each bearing a lone pair of electron are more stable due to resonance.

Triplet Carbenes are more stable than singlet Carbene.