Nucleophillic Substitution reaction

source : slideshare.net
Nucleophilic substitution reaction-
- A nucleophile is an the electron rich species that will react with an electron poor species
- A substitution implies that one group replaces another.
Nucleophilic substitution reactions occur when an electron rich species, the nucleophile, reacts at an electrophilic saturated C atom attached to an electronegative group , the leaving group.
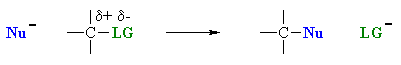
SN1 Mechanism or Unimolecular Nucleophilic Substitution –
S means Substitution , N means Nucleophilic & 1(one) for unimolecular. Unimolecular reactions are those reactions in which rate determining step is unimolecular.
In an SN1 reaction, the rate determining step is the loss of the leaving group to form the intermediate carbocation. The more stable the carbocation is, the easier it is to form, and the faster the SN1 reaction will be.
In SN1 mechanism , reaction takes place in two steps-
In first step , slow heterolytic fission of alkyl halide (one molecule) takes place & alkyl carbonium ion is formed.The reaction is unimolecular because first step is slow step & it is rate determining step.Only one molecule of alkyl halide takes part in slow step.

Rate of reaction ∝ [Alkyl halide]
The reaction is first order reaction.
In second step ,carbonium ion combines with nucleophile OH– (obtained from aqueous alkali) to form alcohol.
R+ + OH– ——-> ROH (alcohol) [ FAST STEP ]
The hydrolysis of tertiary butyl halide takes place by SN1 mechanism.
Reactivity order : (CH3)3C- > (CH3)2CH- > CH3CH2– > CH3–
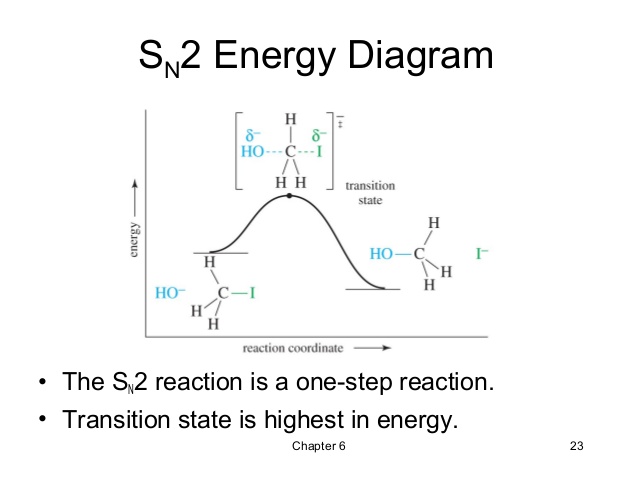
SN2 Mechanism or Bimolecular Nucleophilic Substitution –
S means Substitution , N means Nucleophilic & 2 (two) for bimolecular.
In SN2 mechanism , one molecule of alkyl halide & one molecule of aq. alkali combine together to form transition state .This is slow & rate determining step. So reaction is bimolecular .

Rate of reaction ∝ [Alkyl halide] [OH–]
The reaction is second order reaction.
The transition state breaks with the formation of alcohol. Hydrolysis of methyl bromide takes place by SN2 mechanism.
Reactivity order : CH3X > Primary > secondary > tertiary
Factors affecting reactivity in substitution reactions-
1) Structure of alkyl group-
In SN2 mechanism , the order of reactivity of alkyl halides is,
CH3X > Primary > secondary > tertiary
When H – atoms of methyl halide are replaced by bulkier alkyl group , then it is more difficult to form transition state (due to steric hinderance of alkyl groups).
In SN1 mechanism, the order of reactivity of alkyl halides is,
Tertiary > secondary > primary > CH3X
because tertiary carbonium ions are more stable.
2) Nucleophile-
The nucleophilicity of a reagent is defined as its ability to donate an electron pair to carbon & the nucleophilicity increases with the increase in the size of the atom having lone pair of electron.
The transition state of SN2 reaction involves nucleophile & rate of reaction depends upon the reactivity of the reagent (nucleophile).
I– > Br– > Cl–
Atoms of bigger size are more polarisable and better nucleophile.
In SN1 reaction the nucleophile reacts with carbonium ion in second step ( which is fast step), so nucleophilicity of the reagent is of less importance.
3) Solvent-
SN1 reaction starts with the ionization of a neutral molecule and thus it is facilitated with good ionization solvents.
In SN2 reaction, an anion reacts with neutral molecule, hence a good ionising solvent slows down the reaction because it stabilizes the reaction more effectively.