Elimination reaction

source : tutorvista.com
Elimination reaction (E1 & E2)
An elimination reaction is a type of reaction in which two atoms or groups are removed from a molecule in either one or two-step mechanism . The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction.
Ex-
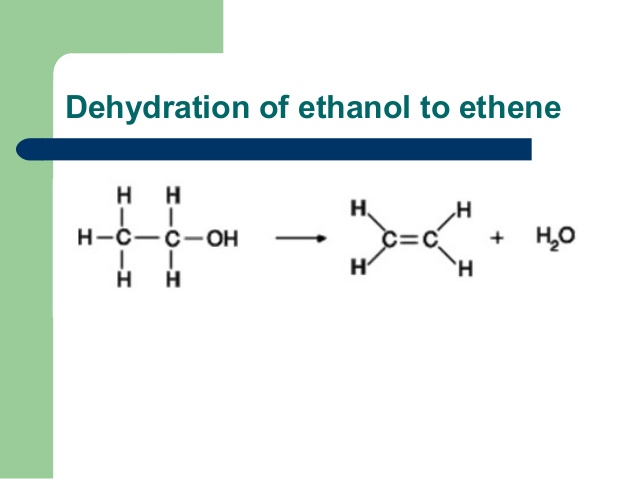
When alkyl halide is heated with alc. KOH, then alkene is obtained. This reaction is elimination reaction because one water molecule is eliminated.
CH3-CH2-Br + KOH ………..> CH2=CH2 + KBr + H2O
(Ethyl bromide) (Potassium hydroxide) Ethylene or Ethene
E1 mechanism or unimolecular elimination mechanism :
In E1 mechanism the reaction takes place in two steps i.e, ionization and deprotonation. In first step heterolytic fission of alkyl halide takes place and alkyl carbonium ion is formed .The rate of reaction is influenced only by the concentration of the alkyl halide because carbocation or carbonium ion formation is the slowest step. Therefore it is rate determining step & reaction is unimolecular .

Rate of reaction ∝ [Alkyl halide]
The reaction is first order reaction.
In second step, the carbonium ion combines with one molecule of alc. KOH to give alkene with the elimination of one water molecule.

Highly substituted carbonium ions are more stable than methyl or primary carbonium ion. Therefore, stability give two step E1 mechanism. E1 typically takes place with tertiary alkyl halide.
E2 Mechanism or Bimolecular elimination mechanism:
E2 stands for bimolecular elimination. The reaction involves a one-step mechanism in which carbon-hydrogen and carbon-halogen bonds break to form a double bond (>C=C< means pi bond)
In E2 mechanism one molecule of alkyl halide and one molecule of alc. KOH combines together to give alkene. This reaction is bimolecular. One molecule of alkyl halide and one molecule of alc. KOH take part in the reaction. This is slow step and rate determining step.
![]()

This reaction is second order reaction.