Catalysis : short note-
source : gcy linder.com
Catalysis : short note-
Q. What is the difference between enzyme & ordinary catalyst
Enzyme Catalyst-
1. These are produced in living cell
2. These are nitrogenous compounds having high molecular weight. Enzymes are proteins.
3. The activity of enzyme is affected by pH therefore enzymes are more active at optimum pH
4. Their activity is destroyed by UV-rays
5. Enzymes are very specific in nature.
6. Generally enzymes are colloidal in nature
Ordinary Catalyst-
1. These are mostly found in nonliving substances.
2. Catalysts may be metals or their compound & acids or base.
3. There is no effect of pH.
4. There is no effect of UV-rays on catalyst.
5. Catalysts are specific in their acion.
6. These are not colloidal in nature.
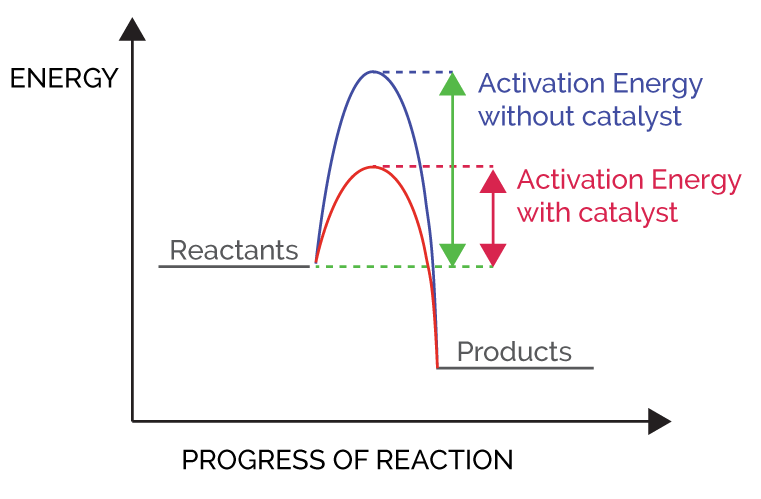
Effect of Catalyst on reaction rates-
Catalyst provides entirely new path for the reaction in which the reactants are converted into products quickly. It is believed that catalyst forms a new activated complex of lower potential energy. It means activation energy becomes lower for the catalysed reaction than that for uncatalysed reaction.
Activation energy-
The excess energy (over & above the average energy of the reactants) required by reactants to under go chemical reactions is called activation energy (Ea). It is equal to the difference between the threshold energy needed for the reaction & the average Kinetic energy of all the reacting molecules .
Ea ( Activation energy) = E(threshold) – E(reactants)
Promoters-
Those substances which do not themselves acts as catalyst but it increases the activity of catalyst are known as activators or promoters.
Ex. 1 : Iron is used as a catalyst in Haber’s process for the manufacture of NH3. If small amt. of Mo is mixed with Iron then catalytic power of Fe is increase. Hence Mo acts as promoter.
N2 + 3H2 —–> 2NH3
mixture of ( K2O + Al2O3) act as promoter
2. Nickel acts as catalyst in the manufacture of Vegetable ghee from Vegetable Oil. Telurium acts as a promoter.
Vegetable oil + H2 ———> Vegetable Ghee.
Catalytic Poisons-
Catalytic poisons are substances which decreases or destroy the activitiy of a catalyst .
Ex.
1. If small amount of As2O3 is present along with Pt in the manufacture of H2SO4 by contact process. Then catalytic power of Pt is greatly decrease. Therefore As2O3 acts as a catalytic poison2. Catalytic power of Fe catalyst is almost destroyed in presence of H2S gas . Therefore in the manufacture of NH3 by Haber’s process , activity of Fe catalyst is destroyed by H2S . Therefore it acts as poison for Fe Catalyst.3. In the conversion of acetylene into ethylene Lindlar catalyst [(Pd-CaCO3(CH3COO)2Pb] is used. Lead acetate acts as poison for Pd catalyst.
Difference between -ve catalyst & catalytic poison-
Negative catalyst decreases the rate of reaction but catalytic poison decreases or destroy the activity of catalyst.
Read more articles at chemistryonline.guru