Catalyst : properties

source : chemicals array our friend.com
Catalyst : properties-
1. Unchangeability of the Catalyst :
A Catalyst remains unchanged in mass & chemical composition at the end of the reaction. But there may be little change in its physical state.
Ex.
MnO2 is used as a catalyst in the preparation of O2 from KClO3 . MnO2 is solid, but during the reaction particle size of MnO2 is decreased.
2. Small quantity of the catalyst-
Usually small quantity of the catalyst is sufficient to carry out the reaction because catalyst is not used up during the reaction.
Ex.
Only one gram of colloidal Pt is sufficient to increase the rate of decomposition of 106 litre of H2O2.
Exceptions : In many homogeneous catalytic reactions, the rate of reaction is proportional to the concentration of catalyst.
In some heterogeneous reactions , the rate of reaction is proportional to the surface area of solid catalyst.
3. Initiation of reaction-
A catalyst doesn’t start a reaction. A catalyst only increases or decreases the rate of reaction. It doesn’t start the reaction.
4. Catalyst & the nature of product:
Generally catalyst don’t change the nature of product. Some exceptions are :

5. Catalysts are specific in their action-
A substance acts as a catalyst for a particular reaction. It is not necessary that it will act as a catalyst for any other reaction. Hence catalysts are specific in their action.

6. Effect of Temperature-
Activity of a catalyst is maximum at a definite temperature which is known as optimum temperature.
7. A Catalyst doesn’t Change the state of equilibrium of a reaction-
A catalyst increases the rate of both the forward & backward reaction equally. Hence catalyst doesn’t influence the state of equilibrium of a reversible reaction but equilibrium is attained earlier.
8. Activity of Finely divided Catalyst is Greater –
It is because surface area of finely divided catalyst is greater
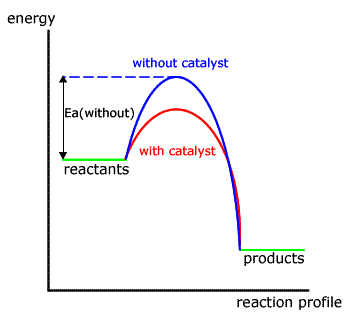
9. A catalyst lowers the activation energy-
According to arrhenious collision theory , reaction occurs on account of effective collisions between the reacting molecules. For effective collision it is necessary that the molecule must possess a minimum amountof energy known as activation energy. The catalyst provides a new path way involving lower amount of activation energy. Hence large number of effective collisions occur in presence of catalyst in comparison to the effective collisions at the same temperature in absence of catalyst. Therefore the presence of a catalyst makes the reaction to go faster.
Read more articles at chemistryonline.guru