Classification of matter-

Classification of matter-
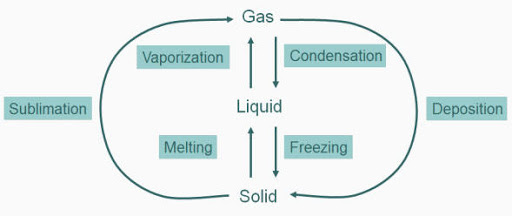
MATTER– It occupies space & possesses mass, it can exist in three physical states – solid, liquid & gas
In solids, particles are held very close to each other in an orderly manner & not freedom of movement. Solids have definite volume & definite shape.
In liquids, particles are close but they can move. Liquids have a definite volume but do not have a definite shape. They can take the shape of the container in which they are kept.
In gases, particles are far apart as compared to solid & liquid & their movement is easy & fast. Gases have neither a definite volume nor a definite shape. They completely occupy the space of the container in which it is kept.

matter
Source: Dreamstime.com
PURE SUBSTANCE– When matter having an invariant chemical composition & distinct properties then it is called a pure substance.
Ex- Copper, silver, gold, water, glucose, sugar, etc.
ELEMENT – Those fundamental substances which can not be separated into simpler substances by chemical methods, is called an element.
Ex- Cu, Au, Pb, Na, etc.
COMPOUND – The substance composed of two or more elements in fixed proportions & can be separated into simpler substances & elements only by chemical methods like electrolysis are called a compound.
Ex- CaCl2,NaBr , NaCl,C6H12O6 etc.
MIXTURE– When matter consists of two or more pure substances that retain their individual identities & can be separated by physical methods such as handpicking, filtration, distillation & crystallisation is called a mixture.
Ex – Mixture of sodium chloride & sugar, mixture of sand & salt, Sugar solution, air
HOMOGENEOUS MIXTURE- Mixture having a uniform composition & properties throughout is called a homogeneous mixture.
Ex- Sugar solution, air
HETEROGENEOUS MIXTURE- Mixture not having uniform composition & properties throughout is called a heterogeneous mixture.
EX- Mixture of salt & sugar, Grain & pulses along with stone pieces.