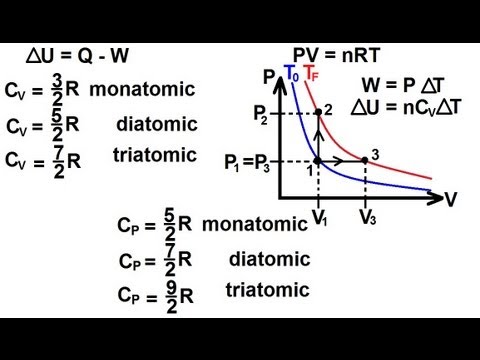
Cp and Cv-

source : SlideShare
Cp and Cv-
Ratio of specific or molar heats (Cp/Cv) –
The molar heat ratio is defined as the ratio of molar heat at constant pressure and molar heat at constant volume. The ratio of specific heat is the same as the ratio of molar heats or heat capacity and is represented by γ.
γ = Cp/ Cv = Cp,m/Cv,m = Cp/Cv
Value of Cp/Cv for mono-atomic gases-
Like He,Ar,Kr,Xe and mercury vapours etc.
γ = (5/2) nR / (3/2) nR
= 5/3
γ = 1.66 or 1.67
Value of Cp/Cv for diatomic gases-
In diatomic gases , heat supplied is used in increasing kinetic energy of the molecules and also in rotational and vibrational energy of atoms with in the molecule. So more heat is needed to raise the temperature of gas through one degree.
So,
Cp,m =( 5/2) R+ x
Cv,m =( 3/2) R+ x
The value of ‘x’ varies with the atomicity of gas molecules .
In case of diatomic gases like H2, O2 , N2 etc. , the value of ‘x’ is,
x=R
γ = [( 5/2) R+R ] / [(3/2) R+R]
= (7/2) R / (5/2) R
γ = 1.4
Value of Cp/Cv for triatomic gases –
In case of triatomic gases like O3 , H2S , C02 etc. , the value of ‘x’ is,
x=3/2 R
γ = [ (5/2) R + (3/2) R] / [(3/2) R + (3/2) R]
γ = 4/3
γ = 1.33
Uses of the ratio Cp/Cv-
It helps ,
- to determine the atomicity of gas molecules.
- by knowing the atomicity and molecular weight, the atomic weight can be calculated.
Atomic weight = Molecular weight/ Atomicity