Cp and Cv-

source : SlidePlayer
Cp and Cv-
Specific heats or molar heats of gases-
‘ The specific heat of a substance is the quantity of heat required to raise the temperature of one gram of substance through one degree C’.
The value of specific heat may be vary depending on the heating conditions of gas, but there are only two values.
(i) Molar heat of a gas at constant volume ‘Cv’ –
It is defined as the quantity of heat in calories required to raise the temperature of one mole of gas through one degree C, at constant volume.
(ii) Molar heat of a gas at constant pressure ‘Cp’ –
It is defined as the quantity of heat in calories required to raise the temperature of one mole of gas through one degree C, at constant pressure.
Chemical calculations are most frequently made on molar basis i.e for one mole of gas the heat required is called molar heat or heat capacity so term molar heat is used in place of specific heat.
Molar heat at constant volume,
Cv,m = Cv x M
M = molecular weight
Molar heat at constant pressure
Cp,m= Cp x M ( for 1 mole)
Similarly molar heat for ‘n’ mole at constant volume,
=Cv x n x M
Because,
Cv x M =Cv,m
Therefore,
Heat capacity at constant volume = Cv,m x n
Heat capacity at constant pressure,
= Cp x n x M
because,
Cp x M =Cp,m
Therefore ,
Heat capacity at constant pressure = Cp,m x n
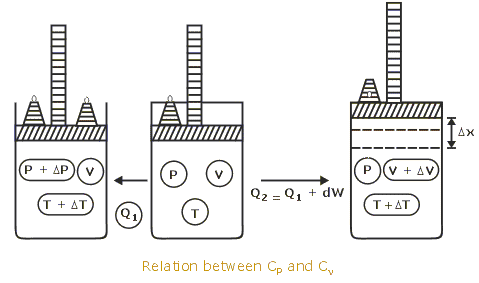
Difference between Cp and Cv :
” When gas is heated at constant volume , no pressure -volume work is done and total heat supplied is used up in increasing the kinetic energy of the molecules.”
” While when the gas is heated at constant pressure the heat is used in increasing the kinetic energy of the molecules and some work is done against the external pressure during expansion.”
Therefore, more quantity of heat is required at constant pressure than at constant volume by an amount equal to mechanical work done and temperature raised by one degree.
Cp = Cv + work done in expansion
Therefore,
Cp> Cv
Cp,m > Cv,m (Molar heat =specific heat x M)
Cp> Cv
M= Molecular weight