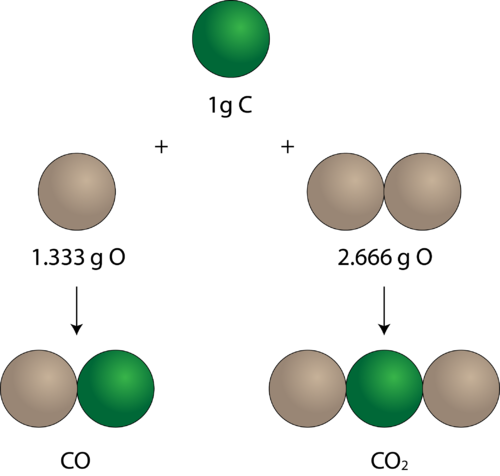
Law of multiple proportion-

source : pinterest.com
Law of multiple proportion-
This law was given by John Dalton. According to this law,
” When two elements combine to form two or more than two compounds, the weights of one of the element which combine with a fixed weight of other,bear a simple whole number ratio.”
OR
” When two elements A and B combine to form more than one chemical compounds then different weights of A, which combine with a fixed weight of B, are in a proportion of simple whole numbers.”
Experimental Verification-
Nitrogen and oxygen combine to form five oxides such as nitrous oxide (N2O) ,nitric oxide (NO),nitrogen trioxide (N2O3),nitrogen tetraoxide (N2O4) and nitrogen pentaoxide (N2O5).
Oxides of Nitrogen Oxygen Fixed wt. of nitrogen Wt.of oxygen combine with 14 gm. of nitrogen
N2O 28 16 14 8
NO 14 16 14 16
N2O3 28 48 14 24
N2O4 28 64 14 32
N2O5 28 80 14 40
Ratio of oxygen in different compounds which combine with same weight of nitrogen
8 : 16 : 24 : 32 : 40
1 : 2 : 3 : 4 : 5
The simple whole number ratio favours Law of multiple proportion.
Problem 1 :
Tin combines with oxygen to form two compounds having the following composition ;
% of Sn % of Oxygen
Compound A 78.77 21.23
Compound B 88.12 11.88
Show that the above data follows the law of multiple proportion.
Solution –
In compound A ,
21.23 part of oxygen combines with 78.77 part Sn
1 part of oxygen combines with = 78.77 x 1 /21.23
= 3.7 part tin
In compound B ,
11.88 part of oxygen combines with 88.12 part Sn
1 part of oxygen combines with = 88.12 x 1 /11.88
= 7.4 part tin
Ratio of Sn with one part of oxygen = 3.7 : 7.4
= 1 : 2
So data follows law of multiple proportion.
Problem 2 :
Carbon combines with hydrogen to form compounds having the following composition ;
% of C % of H
Compound A 75.0 25.0
Compound B 85.7 14.3
Compound C 92.3 7.7
Show that the above data illustrate the law of multiple proportion.
Solution –
In compound A ,
75.0 part of Carbon combines with 25.0 part Hydrogen
1 part of Carbon combines with = 25.0 x 1 / 75.0
= 0.33 part hydrogen
In compound B ,
85.7 part of Carbon combines with 14.3 part Hydrogen
1 part of Carbon combines with = 14.3 x 1 / 85.7
= 0.166 part hydrogen
In compound C ,
92.3 part of Carbon combines with 7.7 part Hydrogen
1 part of Carbon combines with = 7.7 x 1 / 92.3
= 0.08 part hydrogen
Ratio of Hyrogen with one part of Carbon = 0.33 : 0.166 : 0.08
= 4 : 2 : 1
So data follows law of multiple proportion.