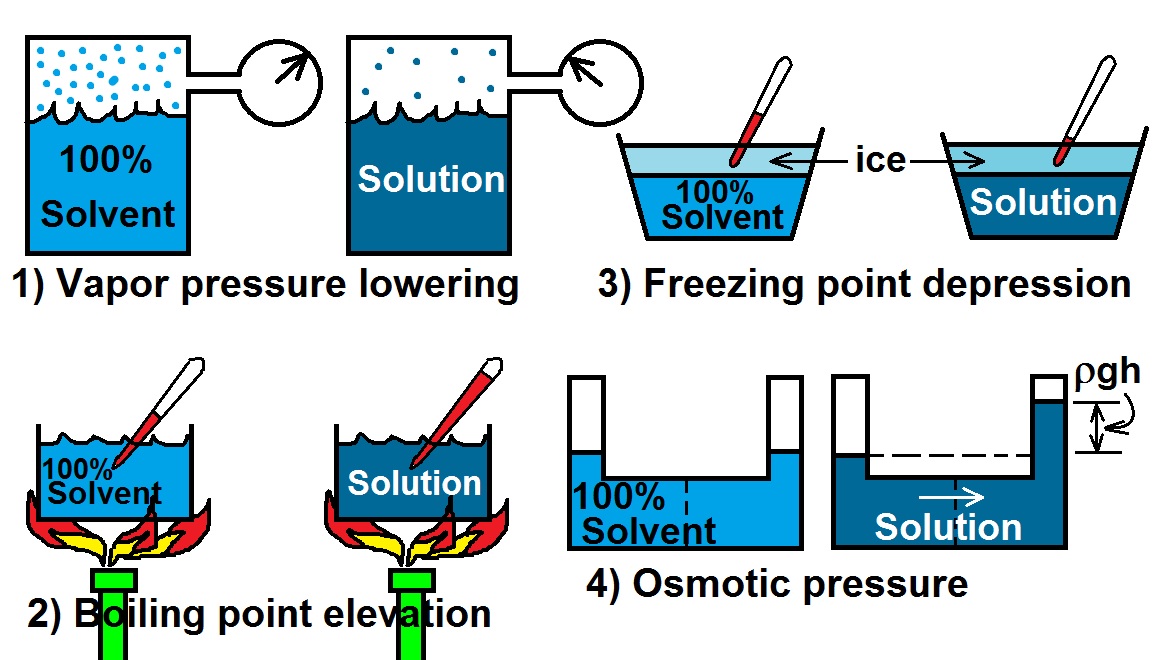
Depression in freezing point

source : Slideplayer.com
Depression in freezing point-Numerical
Question 1) Latent heat of fusion of ice is 80 calorie. Calculate molar and molal depression constant ?
Solution )
Latent heat of fusion ‘L’ = 80 calorie
freezing point of water ‘T’ = 0 + 273 = 273 K
K molar (KM) = 0.02 T2 / L
K molar (KM) = 0.02 x 273 x 273 /80
K molar (KM) = 18.63 K .(per 100 gm )/mole Ans.
K molal (Km) = 0.002 T2 / L
K molal (Km) = 0.02 x 273 x 273 /80
K molal (Km) = 1.863 K Kg /mole Ans.
Question 2) An aqueous has 5 % urea and 10 % glucose by weight . What will be freezing point of this solution ? ( Kf for water = 1.86 K Kg /mole)
Solution )
w urea = 5 gm , w glucose = 10 gm
weight of solution = 100 gm
weight of solvent = weight of solution – weight of solute
weight of solvent = 100 – ( 5 + 10 ) = 85 gm
m of urea ( NH2CONH2) = 14 +2 + 12 +16 + 14 +2 = 60
m of glucose ( C6H12O6 ) = 12 x 6 + 1 x 12 + 16 x 6= 180
ΔTf = ΔTf (urea) + ΔTf(glucose)
ΔTf = [1000 Kf . w(urea)/ m (urea).W ] + [1000 Kf . w(glucose)/ m(glucose).W ]
ΔTf = [1000 x 1.86 x 5 /85 x 60 ] + [ 1000 x 1.86 x 10 / 85 x 180]
ΔTf = 1.824 + 1.216
ΔTf = 3.04
Depression in freezing point (ΔTf ) = freezing point of solvent – freezing point of solution
3.04 = 0 – freezing point of solution .
freezing point of solution = – 3.040C Ans.
Question 3 ) The freezing point of a solution containing 0.2 gm of acetic acid in 20.0 gm benzene is lowered by 0.450C . Calculate the degree of association of acetic acid in benzene . Assume acetic acid dimerises in benzene . Kf of benzene = 5.12 K Kg /mole.
Solution )
w =0.2 gm , W = 20 gm , ΔTf = 0.45 , Kf =5.12 K Kg/mole
m = 1000 Kf . w /ΔTf .W
m = 1000 x 5.12 x 0.2 / 0.45 x 20
m(observed) = 113.78
2CH3COOH ⇌ (CH3COOH)2
moles before association 1 0
moles after association ( 1 – ∝) ∝ /2
∝ = degree of association
m (normal) / m(observed) = Total moles after association / Total moles before association
m(normal ) of CH3COOH = 12 + 3 + 12 + 16 + 16 + 1 = 60
60 / 113.78 =[ ( 1 – ∝) + ∝ /2] / 1
∝ = 0.945 Ans.
% of ∝ =0.945 x 100
% of ∝ = 94.5 % Ans.
Question 4) When 3.24 gm of mercuric nitrate Hg (NO3 )2 is dissolved in 1 Kg of water , the freezing point of the solution is found to be – 0.0558 0C. When 10.84 gm HgCl2 is dissolved in 1 Kg of water , the freezing point of the solution is – 0.07440C. Kf = 1.86 K Kg /mole. Will either of these dissociate and if so what is degree of dissociation ?
Solution )
(i) For mercuric nitrate Hg (NO3 )2 ,
w = 3.24 gm , W = 1 Kg = 1000 gm , freezing point of solution = – 0.05580C , Kf = 1.86 K Kg / mole
freezing point of solvent (water) = 00C
Depression in freezing point (ΔTf ) = freezing point of solvent – freezing point of solution
= 0 – ( -0.0558)
ΔTf = 0.0558
m = 1000 Kf . w /ΔTf .W
m = 1000 x 1.86 x 3.24 / 0.0558 x 1000
m(observed)of Hg (NO3)2= 108
m (normal) of Hg (NO3)2 = 200 + (14 + 16 x 3)2 =324
Hg (NO3)2 ⇌ Hg++ + 2 NO3–
moles before dissociation 1 0 0
moles after dissociation ( 1 – ∝) ∝ 2∝
Total moles after dissociation = 1- ∝ + ∝ + 2 ∝ = 1 + 2 ∝
Total moles before dissociation = 1 + 0 + 0 = 1
m (normal) / m(observed) = Total moles after dissociation / Total moles before dissociation
324 / 108 = (1 + 2 ∝)/ 1
108 + 216 ∝ = 324
216 ∝ = 324 -108
216 ∝ = 216
∝ (degree of dissociation ) = 1
% of ∝ =1 x 100 = 100 %
Hg (NO3)2 dissociates 100 %.
(ii) For mercuric chloride Hg Cl2 ,
w = 10.84 gm , W = 1 Kg = 1000 gm , freezing point of solution = – 0.07440C, Kf = 1.86 K Kg / mole
freezing point of solvent (water) = 00C
Depression in freezing point (ΔTf ) = freezing point of solvent – freezing point of solution
= 0 – ( -0.0744)
ΔTf = 0.0744
m = 1000 Kf . w /ΔTf .W
m = 1000 x 1.86 x 10.84 / 0.0744 x 1000
m(observed) of Hg Cl2= 271
m (normal) of Hg Cl2 = 200 + (35.5 x 2) = 271
There is no dissociation of Hg Cl2 in water because molecular weight
( observed) and molecular weight ( normal ) both are equal ( i-e 271 ) .
∝ (degree of dissociation ) = zero Ans.
Hg (NO3)2 dissociates in water and its degree of dissociation is 100 % Ans.