Fuel

source : chest of books.com
Fuel
Coal gas:
It is a mixture of H2, CO, CH4, C2H4, C2H2, etc. CO2, O2 and N2 is present as impurity.
Percentage composition:
H2 : 45%
CH4 :35%
C2H4 & C2H2 : 4%
CO: 8%
O2 ,N2 & CO2 : 8%
Question: Why is coal gas used as illuminant and fuel?
Solution:
Coal gas is used as illuminant and fuel because unsaturated hydrocarbons like ethylene and C2H2 produces light and heat. Therefore it is called illuminant. Combustion of H2, CH4, CO produces heat only. Therefore it is called fuel.
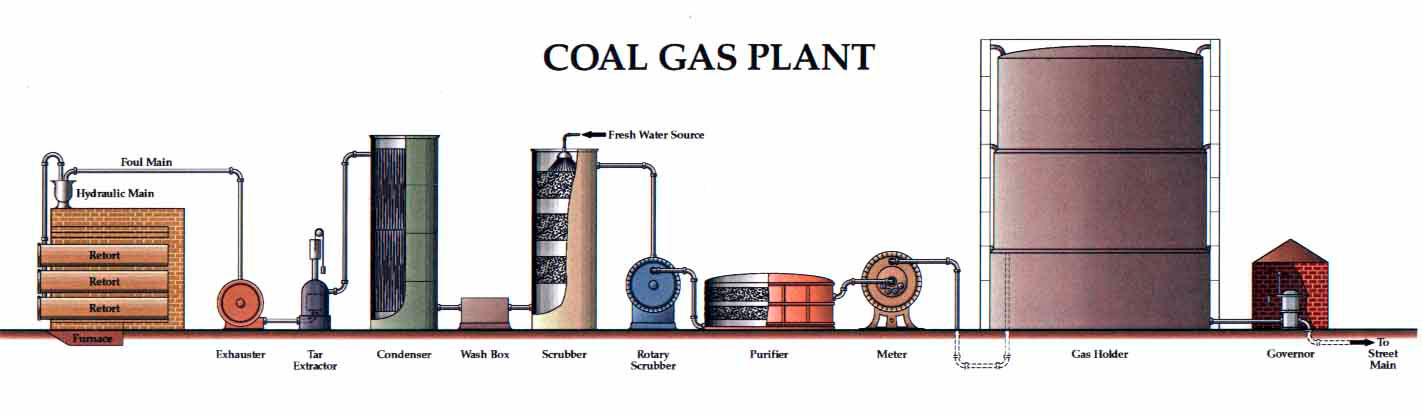
Manufacturing of Coal gas:
Coal is heated in a retort in absence of air it is called destructive distillation. The gas obtained is cooled in an air condenser. NH3 and coal tar condense. Now, gaseous mixture is passed in the tower containing coke. This tower is called washer or scrubber. Water is added in the washer then impurities of NH3 and CO2 are dissolved in H2O and are removed. Some impurities of H2S and HCN are also removed in the washer.
The gases coming out from washer are passed in purifier chamber. In this chamber calcium hydroxide and moist Fe2O3 is kept. In this chamber impurities of H2S, CS2 and HCN are removed. Now the gaseous mixture is passed in stripper. In stripper gaseous mixture is washed with oil then impure benzene, Napthalene are removed. Pure coal gas is checked by meter and collected in the glass holders.
Fe2O3.H2O (moist ferric oxide) +6HCN ——–> 2Fe(CN)3 +4H2O
Fe2O3.H2O (moist ferric oxide) +3H2S ——–> Fe2S3 + 4H2O
Ca(OH)2 +2H2S ———-> Ca(HS)2 (calcium bi sulphide) +H2O
Ca(HS)2 +CS2 (carbon sulphide) —–> CaCS3(calcium thio-carbonate)+H2S
Following byproducts are obtained in the manufacturing of coal gas:
1. Ammonical liquor:
Its aqueous layer contain NH3, ammonium sulphide and other ammonium salt. NH3 is manufactured by ammonical liquor.
2. Coal tar:
It is black viscous liquid. It contains benzene, toluene, naphthalene, phenol etc.
3. Coke:
It is left in the iron retort as residue. It is used in the metallurgy.