Hybridization

source :Chemistry Stack Exchange
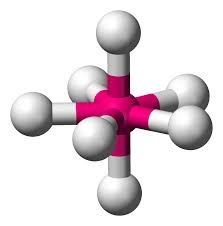
Sp3d3 hybridization :
The mixing of one s, three p and three d- atomic orbitals to form seven equivalent sp3d3 hybrid orbitals of equal energy. This hybridization is known as sp3d3 hybridization.
properties :
- Seven sp3d3 hybrid orbitals are directed towards the corners of a pentagonal bipyramid.
- These are not equivalent hybrid orbitals because five of them are directed towards the corners of a regular pentagon while the remaining two are directed above and below the plane.
- The geometry is pentagonal bipyramidal and bond angle is 720 and 900.
Example : formation of IF7
In IF7 molecule the central atom is I.
53I – 1s2 ,2s2,2p6,3s2,3p6,4s2,3d10,4p6,5s2,4d10,5p5
Seven atomic orbitals (one s, three p and three d orbitals) hybridize to form seven sp3d3 hybrid orbitals. These are singly filled. These hybrid orbitals overlap with singly filled 2pz atomic orbitals of seven F-atoms to form seven I-F sigma bond. Geometry of IF7 is pentagonal bipyramidal and bond angle is 720 and 900.


source : www.adichemistry.com
Hybridization in Xenon fluorides :

source : Slideshare
Geometry of XeF2 is linear due to presence of three lone pair of electrons in sp3d hybrid orbitals . Geometry of XeF4 is square planar due to presence of two lone pair of electrons in sp3d2 hybrid orbitals & XeF6 is distorted octahedral because one lone pair of electron is present in sp3d3 hybrid orbital.