Hybridization
source:chemistry.stackexchange.com
sp3d2 hybridization :
The mixing of one s, three p and two d- atomic orbitals to form six equivalent sp3d2 hybrid orbitals of equal energy. This hybridization is known as sp3d2 hybridization.

source: Chemistry Assignment
Properties :
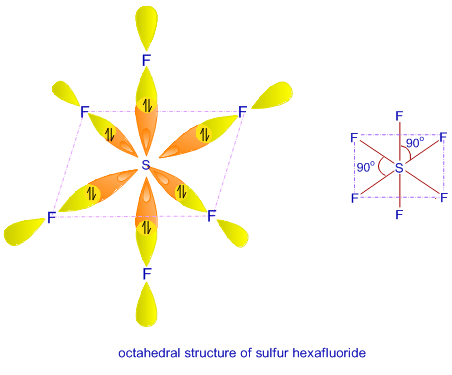
1) sp3d2 hybrid orbitals are directed towards the six corners of regular octahedron.
2) Four out of six hybrid orbitals are lying in one plane while remaining two are directed above and below the plane containing four hybrid orbitals perpendicularly.
3) Geometry is octahedral and bond angle is 900.

source:philschatz.com
source : Tutorvista.com
Formation of SF6 :
In SF6 molecule the central atom in S.
16 S : 1s2,2s2,2p6,3s2,3p4


source : www2.usdcb.on.ca
Six sp3d2 singly occupied hybrid orbitals are directed towards six corners of a regular octahedron. These singly occupied sp3d2 hybrid orbitals overlap with six singly filled 2pz atomic orbitals of six F-atom to form six sigma bonds (S-F) . Geometry is octahedral.

source : Chemistry@TutorVista.com

source : www.adichemistry.com
Other example : PF6– , SiF6— , AlF6— etc.

source : you tube

source : virtantiq.com

source : chemistry@tutorvista.com