Hybridization
source : en.wikipedia.org
sp3d hybridization :
The mixing of one s ,three p and one d-atomic orbitals to form five sp3d hybrid orbitals of equal energy is called sp3d hybridization. These orbitals are called hybrid orbitals. These five orbitals are not equivalent. These are divided into two sets :
a) Equatorial hybrid orbitals :
Three hybrid orbitals are directed towards the corners of an equilateral triangle are called equatorial hybrid orbitals. These are planar and bond angle is 1200.
b) Axial hybrid orbitals :
Two hybrid orbitals are perpendicular to the plane of equatorial hybrid orbital .These are called axial hybrid orbital . They make an angle of 900 with equatorial hybrid orbitals.

source : people.stfx.ca


source : philschatz.com
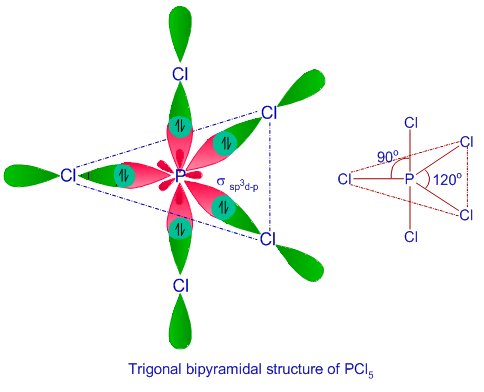
Example: PCl5
In PCl5 molecule the central atom is P.
15P – 1s2,2s2,2p6,3s2,3p3
17 Cl- 1s2,2s2,2p6, 3s2, 3p5

The five sp3d hybrid orbitals are singly occupied . These hybrid orbitals overlap with singly filled 3pz atomic orbital of five Chlorine atom to form five sigma bond (P- Cl).
Geometry of PCl5 molecule is trigonal bipyramidal. Bond angle is 900 and 1200.
Hybridization in SF4 :
In SF4 molecule the central atom in S.
16 S : 1s2,2s2,2p6,3s2,3p4
9F: 1s2,2s2,2p5

source : Chemistry@Tutorvista.com
source : Chemistry@Tutorvista.com
Geometry of SF4 is distorted trigonal bipyramidal due to presence of lone pair of electron.
Hybridization in AB3 type interhalogen compound-
AB3 type interhalogen compounds are ClF3 , ICl3 & BrF3.
17Cl –