Hybridization
source : Chemistryland
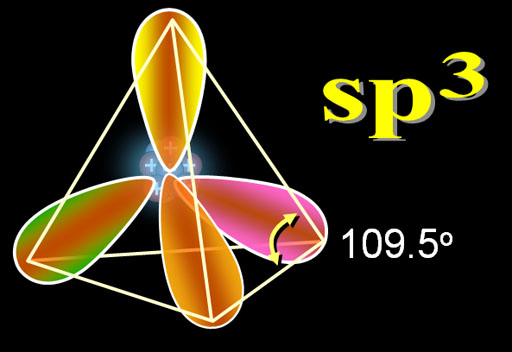
Hybridization – sp3
In this hybridization one s , three p orbital are mixed together and four hybrid orbitals of equal energy are formed. These are called sp3 hybrid orbital and this process is called sp3 hybridization.
Properties of sp3 hybrid orbitals :
- All four sp3 hybrid orbitals are equivalent in shape and energy.
- The four sp3 hybrid orbitals are directed towards the four corners of regular tetrahedron.
- Bond angle is 109028′.
- Geometry is tetrahedral.
source : grandinetti.org
Example: CH4, C2H6,H2O,NH3, NH4+,SO4—,PO43-
Hybridization in Methane & Ethane –
one s and three p orbitals hybridise to form four equivalent sp3 hybrid orbitals. These are singly filled and directed towards four corners of regular tetrahedron . These four sp3 hybrid orbitals form sigma bond with singly filled s-orbital of H- atom and four sigma bonds are formed. Bond angle is 109028′ and geometry is tetrahedral.

source : Chemistry assignment

source : A Plus Topper

source: Chemistry-assignment.com
Hybridization in Ammonia –
7N- 1s2,2s2,2p3
There four sp3 hybrid orbitals of nitrogen atom of ammonia is formed by the overlapping of three half filled orbitals of Nitrogen atom with s-orbital of 3 hydrogen atoms. There remains a full-filled sp3 hybrid orbital.
Geometry of ammonia is pyramidal or distorted tetrahedral due to presence of lone pair. There is lp-bp and bp-bp repulsion.

source : Chemistry Assignment

source : chemistry @ tutorvista.com
Hybridization in water –
8O- 1s2,2s2,2p4
There are four sp3 hybrid orbitals on oxygen atom . Water is formed by the overlapping of two half filled sp3 hybrid orbital of oxygen atom and s orbital of two hydrogen atom. There remain two full filled sp3 hybrid orbital on oxygen atom.
Geometry of water is V-shaped or distorted tetrahedral due to lp-lp , lp-bp , bp-bp repulsion. The bond angle is 104.50.

source: Chemistry@TutorVista.com

source :SparkNotes