isoelectronic

source : slide player.com
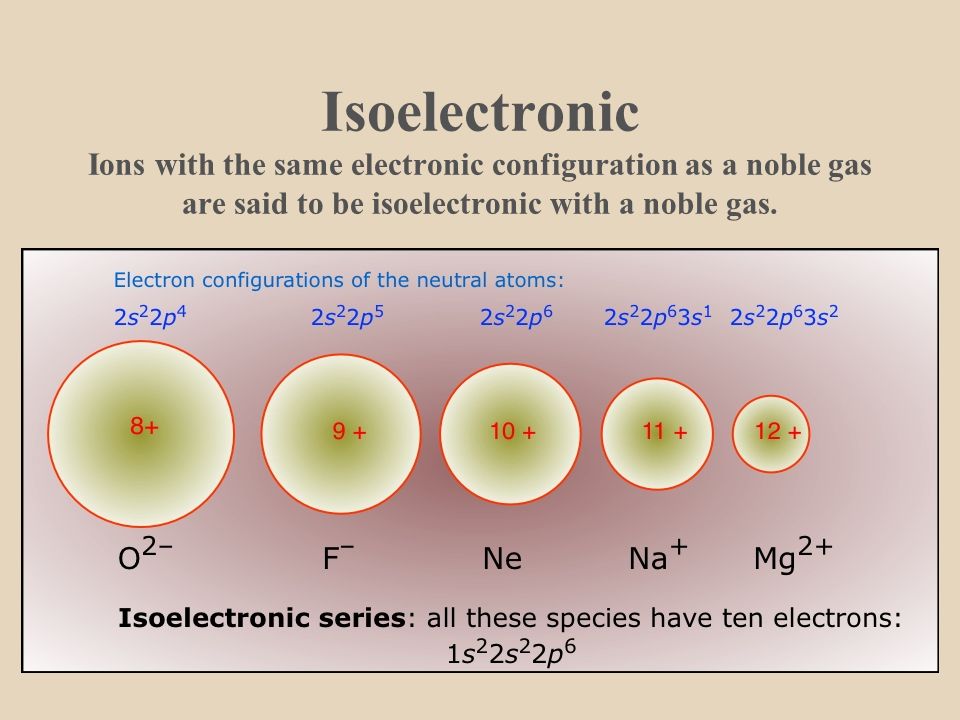
ISOELECTRONIC
Atoms or ions having same number of electrons or same electronic configuration, are called isoelectronic.
Ex. (a) O– –,F–, Na+, Mg++, Al3+, N3–
(b) Ca++, Ar , S– – , K+ , Cl–
(c) He , Be+
(d) Ar , S– –
(e) S– – , P3–
(f) Ne , F–