mass defect

source : h2physics.org
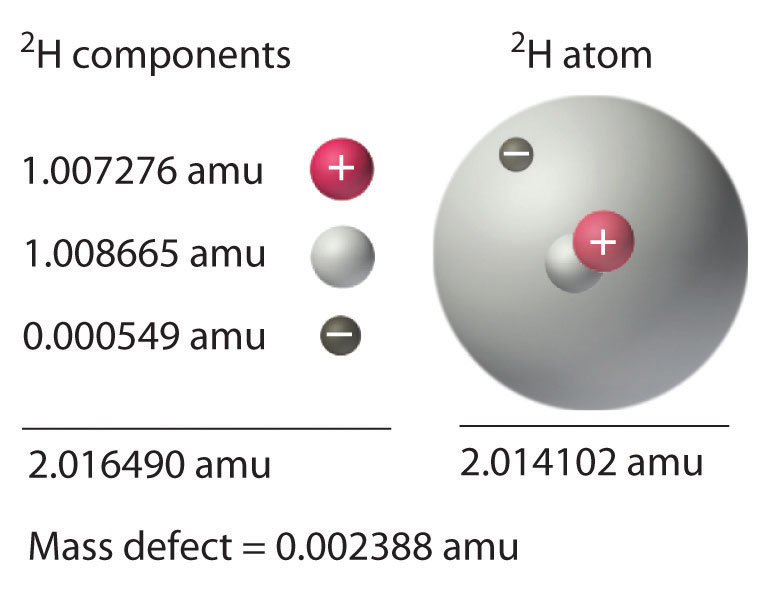
If nucleus is formed by combining neutrons and protons, the mass of nucleus formed will be less than that of the mass of nucleons (mass of proton+mass of neutron) from which it has been formed. This loss in mass is called mass defect.
It is represented by Δm.
Δm=mass of nucleons – actual mass of nucleus
When a nucleus is formed from neutrons and protons, some of the mass is converted into energy according to Einstein equation E=mc2. This is the reason of Δm . It is not the measure of stability of nucleus