Synthesis – Hydrogen Iodide

source : chem wik i.uc davis .edu
Synthesis – Hydrogen Iodide=
The formation of HI from H2 & I2 is represented by eqn
‘a’ moles of H2 & ‘b’ moles of I2 are heated in a sealed tube having ‘v’ litres volume. At equilibrium ‘x’ moles of H2 & x moles of I2 have reacted & 2x moles of HI will be formed
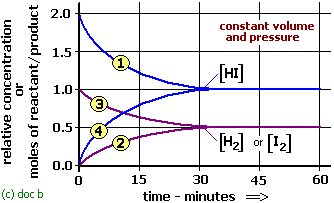
H2 + I2 <—-> 2HI
Initial Conc. (mol./l a/V b/V O
at equi. (mole/l) a-x /V b-x/V 2x /V
A/c to Law of mass action,
Kc=[HI]2/[H2][I2]
Kc =[2X/V]2 /[(a-x)/V] [(b-x)/V]
Kc =4×2 /[(a-x)(b-x)
Factors affecting : Synthesis of Hydrogen Iodide —
Kc =4×2 /[(a-x)(b-x)
- ) Effect of Pressure
Equilibrium expression does not include the volume, therefore Kc is independent of pressure. Thus change of pressure does not alter the final state of equilibrium.
2) Effect of Temp.
The formation of HI is an exothermic reaction. By increasing temperature the value of Kc decreases & the equilibrium is shifted in the backward direction.
Low temperature favours the forward reaction & Synthesis of Hydrogen Iodide increases.
iii) Effect of Concentration-
If H2 or I2 or both is increased, the rate of forward reaction will increase. If conc. of H2 is increased then conc. of I2 will decrease & that of HI will increase & Kc remains constant . If concentration of I2 is increased then conc. of H2 will decrease & that of HI will increase & Kc remains constant.
iv) Effect of adding inert gas
If an inert gas is added at constant volume , the molar concentration do not change, Thus there is no effect on the equilibrium . If an inert gas is added at constant pressure, the volume increases .Kc =4×2 /[(a-x)(b-x) does not include volume & hence there will be no effect on equilibrium on adding gas at constant pressure too.
v) Effect catalyst-
Catalyst increases that rate of forward & backward reaction equally. Therefore Kc remains constant but equilibrium state is attained earlier.