Kohlrausch law

source : AskIITians
Kohlrausch law-
According to this law,
” At infinite dilution , when ionisation is complete, each ion makes its contribution towards equivalent conductance ( or molar conductance) of the electrolyte and at infinite dilution equivalent or molar conductance is given by the sum of the equivalent conductances of two ions (cations and anions).
Λ+m = Λ0+ + Λ0–
Λ0+ = equivalent or molar conductance of cation at infinite dilution
Λ0– = equivalent or molar conductance of anion at infinite dilution
Another form of law,
Λ+m = Λ0+ + + Λ0– –
+ and – are number of cations and anions
Another form of Kohlrausch law,
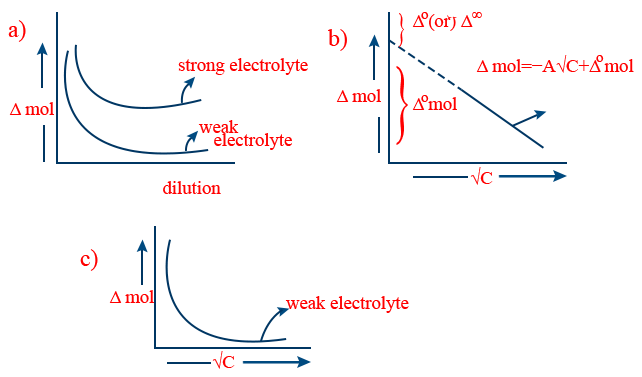
Λm = Λ0m – K√ C
Λm = molar conductance
Λ0m = molar conductance at infinite dilution
K = Kohlrausch constt.
C = concentration
or
Λeq = Λ0eq – K√ C
Λeq = equivalent conductance
Λ0eq = equivalent conductance at infinite dilution
” At low concentration for strong electrolyte , molar or equivalent conductance both vary linearly with square root of conductance”
 +
+ 





