Mixture

source: Quora.com
Mixture-
It is obtained by mixing two or more substances (compound , element ) in any proportion which are not chemically combined is called ‘mixture’.
Ex- Air is a mix. of several gases, Syrup is a mix. of sugar and water.
Properties of Mixture-
1) The components present in mix. do not lose their identity. We can test each component of mixture.
2) The components of mix. can be separated by ordinary or physical methods.
3) Mixture have no definite melting and boiling point.
4) There is no heat change when two components are mixed to form mixture.
Types –
1) Homogeneous Mixture–
It has only one phase .
EX- sugar solution ( sugar dissolved in water),Brine ( sodium chloride dissolved in water),Soda water( CO2 dissolved in water), Alloys like brass, bronze , Air ( mix. of oxygen , nitrogen and other gases).
2) Heterogeneous Mixture-
It has two or more phases. There are visible boundaries of separation between components of mixture.
EX- Mix. of sand in water, Muddy water, Smoke (Mix. of air and carbon particles) , oil in water, Chloroform in water, Carbon tetrachloride in water.
Separation of Mixture –
There are some methods to separate the components of the mix.-
1)Distillation method-
In distillation, separation of volatile components from nonvolatile components or separation of a mix. of volatile components or separation of gas or vapour from a liquid or solid, is possible.
2) Crystallisation –
This technique is used for the separation and purification of substances . In this process mix. is dissolved in the selected solvent ,boiled and filtered. The filterate is cooled and process is repeated several times to get pure component.
3) Sedimentation and Decantation-
This is the process of setting of solid particles from a liquid either to produce a concentrated slurry or to clarify a liquid containing solid particles .If the particles are too small or the difference in the density of solid and liquid is too small then centrifuge machine is used for separation purpose.
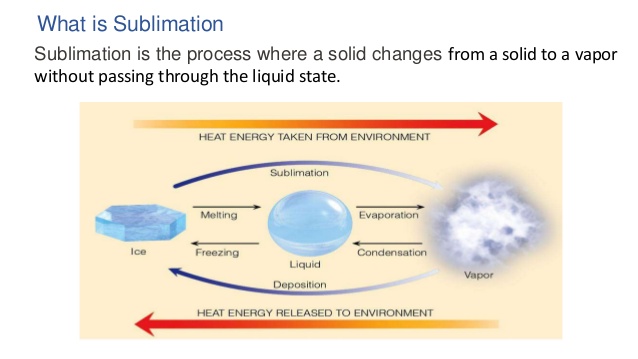
4) Sublimation –
In this process solid phase is directly transformed into vapour form without passing through liquid phase.This method is used in the purification of those substances which have sublimation property.
EX- Iodine , naphthalene, ammonium chloride etc.

source:
5) filtration –
In this process solid particles are separated from suspension through a membrane.