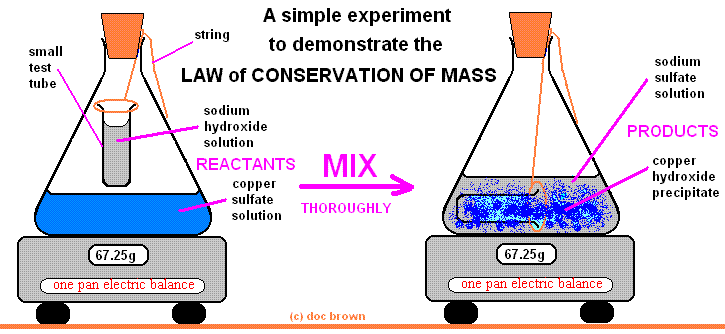
Law of conservation of mass

source : Zigya
Law of conservation of mass-
This law was postulated by French chemist A . Lavoisier.
According to this law,
” Matter is neither created nor destroyed during the chemical reaction, it may change from one form to other.”

source: SlidePlayer
OR
“During any physical or chemical change , the total mass of the products is equal to the total mass of the reactants.”
Heydweiller & Landolt performed different reactions in a closed H-tube so nothing was allowed to escape. It was found that there was hardly any change in the weights of the tube as a result of reaction.
Experimental verification of law by Landolt’s experiment-
Landolt took the solutions of sodium chloride & silver nitrate separately in two tubes of a ‘H’ shaped tube ( Landolt’s tube). The tube was sealed & weighed . The two solutions were mixed thoroughly by shaking the tube then white precipitate of silver chloride is formed.

AgNO3 + NaCl ———> AgCl ( white ppt. of silver chloride)+ NaNO3
After the reaction , the tube was again weighed. It was observed that the weight remains unchanged before and after the reaction.
This verify the law of conservation of mass.
Numerical problems-
Q – 1.7 gm. of AgNO3 dissolved in 100 gm. of water. 0.585 gm. of NaCl dissolved in 100 gm. of water, is added to it & shake it. 1.435 gm. of AgCl & 0.85 gm. of NaNO3 are formed. Justify that the data follows law of conservation of mass.
Solution –
mass of AgNO3 = 1.7 gm.
mass of NaCl = 0.585 gm.
mass of AgCl = 1.435 gm.
mass of NaNO3 = o.85 gm.
AgNO3 +NaCl ——–> AgCl +NaNO3
Total mass of reactants =Mass of AgNO3 + mass of NaCl
= 1.7 +0.585
Total mass of reactants = 2.285 gm.
Total mass of products =Mass of AgCl + mass of NaNO3
= 1.435 + 0.85
Total mass of products = 2.285 gm.
Total mass of reactants = Total mass of products
or
Total mass before reaction = Total mass after reaction
2.285 =2.285
So data follows law of conservation of mass.
Q – When 4.2 gm. of NaHCO3 is added to a solution of CH3COOH weighing 10.0 gm. , it is observed that 2.2 gm. of CO2 is released to the atmosphere. The residue left is found to weigh 12.o gm. Show that these observations are in favour of the law of conservation of mass.
Solution –
Mass of NaHCO3 = 4.2 gm.
Mass of CH3COOH =10.0 gm.
Mass of CO2= 2.2 gm.
Mass of residue = 12.0 gm.
NaHCO3 + CH3COOH ———–> CH3COONa + H2O + CO2
Total mass of reactants =Mass of NaHCO3 + mass of CH3COOH
= 4.2 + 10.0
Total mass of reactants = 14.2 gm.
Total mass of products =Mass of residue [ mass of CH3COONa & H2O]+ mass of CO2
= 12.0 + 2.2
Total mass of products = 14.2 gm.
Total mass of reactants = Total mass of products
or
Total mass before reaction = Total mass after reaction
14.2 =14.2
So data follows law of conservation of mass.