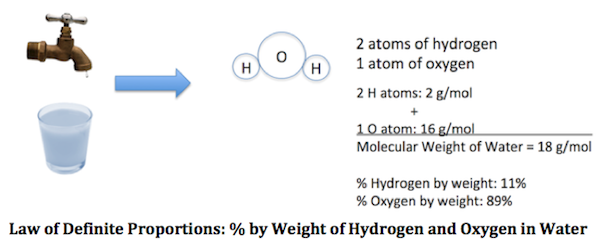
Law of constant proportion
source : Slide share
Law of constant proportion or definite composition-
This law was stated by Louis Proust. According to this law,
‘ A pure chemical compound always contains same elements combined together in the same definite proportion by mass.’
Experimental Verification-
Carbon dioxide can be obtained by so many methods like,
(i) By respiration ( as byproduct )
(ii) By burning coal-
C + O2 ——> CO2
(iii) By heating lime stone-
CaCO3 ———> CaO + CO2
(iv) By heating Sodium bicarbonate-
2NaHCO3 ——>Na2CO3 +H2O + CO2
(v) By the reaction of Dilute Hydrochloric acid & Calcium carbonate-
2CaCO3 + 2HCl ——–> CaCl2 + H2O + CO2
It has been observed that CO2 obtained by every method contains,
C : O
12 : 32
3 : 8 (by weight)
Numerical problems –
problem 1)
(a) 1.08 gram of Cu- wire was allowed to react with HNO3. The resulting solution was dried and ignited when 1.35 gram of CuO was obtained.
(b) In another experiment 2.3 gram of CuO was heated in presence of H2, then 1.84 gram of Cu is obtained.
Show that the above data follows law of constant proportion .
Solution-
Cu + HNO3 ——–> Cu(NO3)2 ———–> CuO
1.08 gram 1.35 gram
a) 1.35 gram CuO contains Cu = 1.08 gram
100 gram CuO contains Cu = 1.08 X100/1.35 =80 gram
% of Cu in CuO =80%
% of O in CuO =(100-80) =20%
b) CuO + H2 ——> Cu + H2O
2.3 gram 1.84 gram
2.3 gram CuO contains Cu = 1.84 gram
100 gram CuO contains Cu = 1.84 X100/2.3 =80 gram
% of Cu in CuO =80%
% of O in CuO =(100-80) =20%
% of Cu in both the cases is same .So data follows law of constant proportion.
problem 2-
1.375 gram CuO was reduced by H2 & 1.098 gram Cu was obtained . In another experiment 1.178 gram of Cu was dissolved in HNO3 & the resulting Cu – nitrate was converted into Cu by heating . The weight of CuO formed was 1.476 gram.
Show that these results prove the law of constant proportion or composition.
Solution –
i) CuO + H2 —–> Cu + H2O
1.375 gram 1.098 gram
1.375 gram CuO contains Cu = 1.098 gram
100 gram CuO contains Cu = 1.098 X100/1.375 = 79.8 gram = 80 gram
% of Cu in CuO =80%
ii) Cu + HNO3 ——–> Cu(NO3)2 ———–> CuO
1.178 gram 1.476 gram
1.476 gram CuO contains Cu = 1.178 gram
100 gram CuO contains Cu = 1.178 X100/1.476 = 79.8 = 80 gram
% of Cu in CuO =80%
% of O in CuO =(100-80) =20%
% of Cu in both the cases is same .So data follows law of constant proportion.
Problem 3-
Copper sulphate crystals contain 25.45 % Cu & 36.07 % H2O. If the law of constant proportion is true then calculate the weight of Cu required to obtain 40 gram of crystalline copper sulphate ( CuSO4.5H2O)
Solution-
100 gram crystalline copper sulphate ( CuSO4.5H2O) contains Cu = 25.45 gram
40 gram crystalline copper sulphate ( CuSO4.5H2O) contains Cu = 25.45 X 40 /100=10.18 gram Ans.