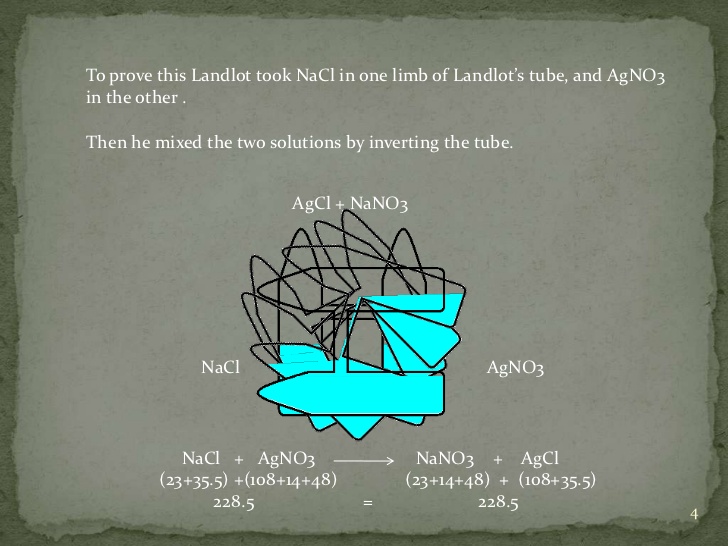
Law of reciprocal proportion

source : slideshare.net
Law of reciprocal proportion or Law of Equivalent proportion –
Richter gave this law.According to this law,
” The weights of the two or more substances which separately react with same weight of a third substance are also the weights of these substances which react with each other or in a simple multiple of them. “
Ex – Hydrogen combine with sulphur and oxygen to form compounds H2S and H2O respectively.
In H2S 2 parts hydrogen 32 parts sulphur
In H2O 2 parts hydrogen 16 parts oxygen
According to the law of reciprocal proportion sulphur should combine with oxygen in the ratio of 32 : 16
( 2 : 1) or a simple multiple of it.
In SO2,
sulphur and oxygen combine in the ratio of 32 : 32 or 1 : 1.
The two ratios are related to each other as 2/1 :1/1 = 2:1
This is a simple multiple ratio.
In SO3,
sulphur and oxygen combine in the ratio of 32 : 48 or 2 : 3
The two ratios are related to each other as 2/1 : 2/3 = 3 : 1
This is a simple multiple ratio.
Question 1) H2S contains 94.11 % sulphur, water contains 11.11 % hydrogen and SO2 contains 50 % oxygen . Show that the results are in agreement with law of reciprocal proportions.
Solution )
In SO2 ,
mass of oxygen = 50 gm
mass of sulphur = 100 – 50 = 50 gm
In H2O,
mass of hydrogen = 11.11 gm
mass of oxygen = 100 – 11.11 = 88.89 gm
Let us fix the mass of oxygen to 1 gm.
In H2O ,
88.89 gm oxygen combine with hydrogen = 11.11
1 gm oxygen combine with hydrogen = 11.11 /88.89 = 0.125 gm
In SO2,
50 gm of oxygen combine with sulphur = 50
1 gm of oxygen combine with sulphur = 50 / 50 = 1.0 gm
The ratio of masses of Hydrogen and sulphur which combine with the fixed mass of oxygen ( 1.0 gm) is ,
0.125 : 1.0 or 1 : 8
In H2S,
mass of sulphur = 94.11 gm
mass of hydrogen = 100 = 94.11 = 5.89
The ratio of masses of Hydrogen and sulphur in H2S is ,
5.89 : 94.11 = 1 : 16
Now comparing the ratio, 1/8 : 1/16= 2 : 1
This is a simple whole number ratio.
Hence given data follows law of reciprocal proportions.