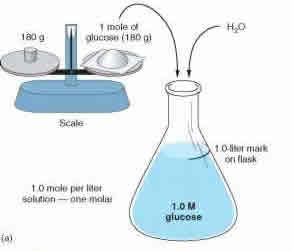
Normality , Molarity , Molality

source : solution- definition. molarity.unit conversion .flow chart and ….
Solved problems of Normality , Molarity , Molality-
Q. 1 ) 49 grams of H2SO4 are present in 100 ml aqueous solution. What is the molarity of H2SO4 ?
Solution : w=49 gm. ,V = 100 ml. =0.1 liter , m of H2SO4 =2 +32+16 x 4=98
M =w/ mV(l)
=49/(98 x 0.1)
M= 5 mole / liter
Q.2 ) An aqueous solution of HCl is 38% by mass & its density is 1.19 gm/ml. calculate the molality & molarity of solution.
Solution :
Mass of solute ‘w’ = 38 gram
Mass of Solution= 100 gram
Mass of Solvent ‘W’= 100-38
= 62 gm = 0.062 kg
m of HCl =1 +35.5 =36.5
molality = w/ mW(Kg.)
=38/(36.5 x 0.62)
molality = 16.79 mole /Kg.
Volume =mass /density=100/1.19 =84 ml. =0.084 liter
M = w/ mV(l)
=38 /(36.5 x 0.084)
Molarity = 12.39 mole/liter
Q.3) 8 grams of solute are dissolved in 10 liter of the solution. Calculate the concentration in ppm.
Solution :
w =8 gm. =8 x 1000 =8000 mg.,V =10 liter
concentration in ppm =weight of solute (mg.)/volume of solution (l)
8000/10 =800 ppm
concentration = 800 ppm
Q.4 ) A mixture of C2H5OH & water contains 54% water by weight. Calculate the mole fraction of alcohol & Water in this mixture ?
Solution :
w of H2O = 54 gm.
w of C2H5OH = 100-54 = 46 gm.
no. of moles of water = 54/18
=3
no. of moles of alcohol = 46/46 = 1
total moles =3 +1 = 4
Xw = nw /total moles =3/4=0.75
mole fraction of water =0.75
X ethyl alcohol =n ethyl alcohol/total moles =1/4=0.25
mole fraction of ethyl alcohol =0.25
Q.5) The density of a 2.03 M solution of acetic acid (molecular weight = 60) in water is 1.017 gm/ml. Calculate molality of the solution.
Solution:
S = Molecular weight × Molarity
= 60 x 2.03
= 121.80 gm/l
Mass of solute (w) = 121.80 gm
v = 1 liter = 1000 ml
mass of solution = V×d = 1000×1.017
= 1017 gm
mass or weight of solvent =1017-121.80=895.2 gm. =0.8952 Kg.
Molality = w/mW(Kg.)
= 121.8 /(60 x 0.8952)
Molality =2.27 mole/Kg.
Q.6) 4 gms of NaOH is dissolved in 200 ml of aqueous Solution. What will be the molarity of this solution ?
Solution :
w =4 gms. , V=200 ml = 0.2 L , m = 23 +16 +1 = 40
Molarity =w /mV (L)
=4 /(40 x 0.2)
M =0.5 mole /liter
Q.7) Calculate the quantity of Na2CO3 required to prepare 250 ml , M/20 solution.
Solution :
w=? , m= 23 x 2+12 +16 x 3= 106 ,V =250 ml =0.25 L
Molarity =w /mV (L)
1/20 =w/ 106 x 0.25
w =1.325 gm.
Q.8) A solution contains 410.3 grams of H2SO4 per liter of Solution at 200 C. If the density is 1.243 gm/ml. What will be its Molarity & Molality.
Ans.
w=410.3 gm.
V= 1 L
m = 2 x 1+32 +16 x 4= 98
Molarity = w/m V (l)
=410.3/98 x 1
Molarity =4.187 mole/liter
Mass of Solution = Volume of Solution × Density
= 1000 ×1.243
= 1243 gm.
weight of solvent = 1243 -410.3 = 832.7 gm = 0.8327 Kg.
Molality = w/mW(Kg.)
=410.3/98 x 0.8327
Molality = 5.028 mole/Kg.
Q. Calculate molarity of pure water. (density =1 gm/ml)
Solution =
Let,volume be 1000 ml
Mass of H2O = 1000×1=1000 gm.
V = 1000 ml. =1 liter
m =2 x 1 +16 =18
Molarity =w/m V(L)
=1000/18 x 1
Molarity=55.56 mole/Kg.