Ostwald dilution law

source : slide player.com
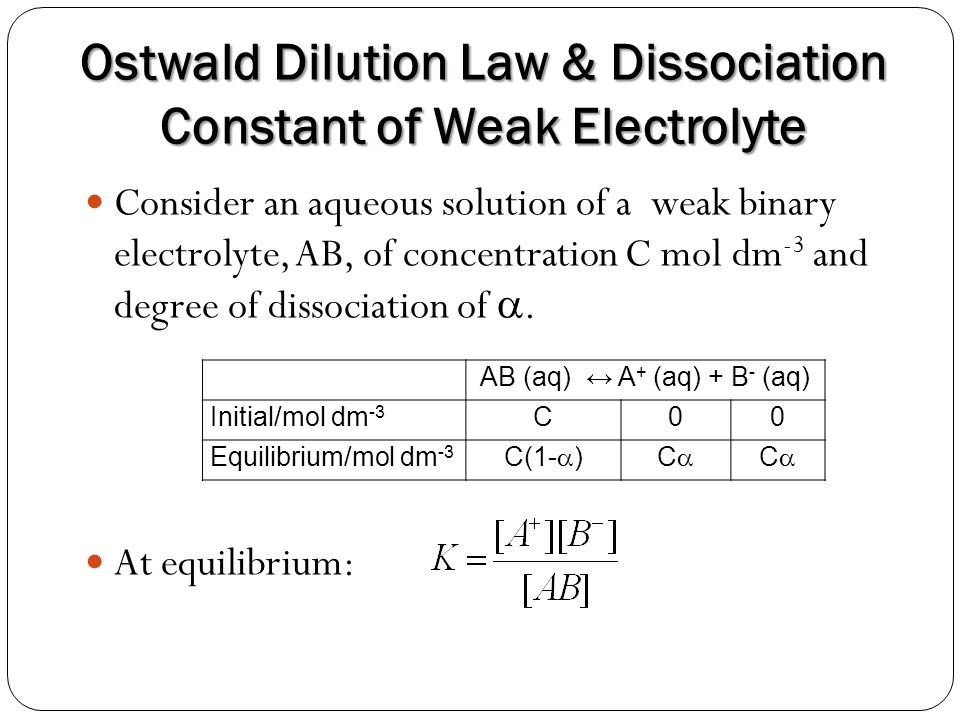
Ostwald dilution law is an application of law of mass action for dissociation equilibrium of weak electrolyte. Consider a weak binary electrolyte,
AB <——> A+ + B–
(Weak Electrolyte)
Moles before dissociation (t=0) 1 0 0
Moles after dissociation (At time ‘t’) 1-α α α
α is degree of dissociation of weak electrolyte ‘AB’ & ‘C’ is the concentration of AB in mole/litre then
[AB] = C(1–α)
[A+] = Cα
[B–] = Cα
At equilibrium
kα = [A+] [B+]/[AB] = (Cα)*(Cα)/C(1-α)
kα =Cα*α/(1-α)
Ka= dissociation constt. of ‘AB’
For weak electrolyte, α<<<1
So 1–α
Ka=Cα2
α=ka/c =
ka.V
“Degree of dissociation of weak electrolyte is directly proportional to square root of dilution.”
Limitations of ostwald Dilution Law-
- This law is applicable only for weak electrolyte
- This law is not applicable for strong electrolytes because strong electrolytes completely ionize at all dilution.