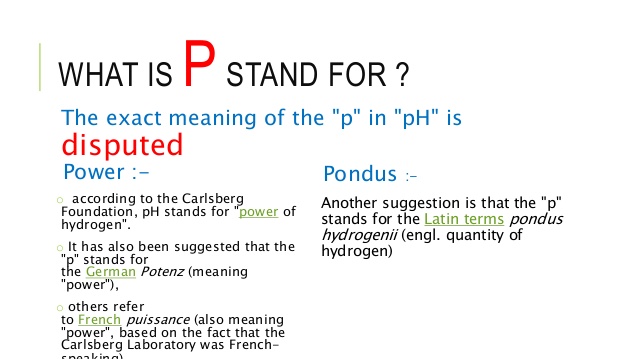
Power of hydrogen ion

source : ontrack-media.net
Power of hydrogen ion ‘pH’ –
Question 1 ) Calculate [H+] and [OH–] concentration in a solution of pH =5 ?
Solution)
pH =5
[H +] = 10 – pH =10-5 gm ion /litre Ans.
[H +] [OH –] = 10 -14
[OH –] = 10 -14 / [H+]
[OH –]= 10 -14 / 10 -5 = 10 -9
[OH –]= 10 -9 Ans.
Question 2) A solution contains 0.0365 gm- ion /litre HCl. Calculate [H+] and pH of the solution ?
Solution)
S = 0.0365 gm /litre
molecular weight of HCl = 1 + 35.5 = 36.5
HCl is mono basic acid . So basicity = 1
E of HCl = molecular weight / basicity of acid
E = 36.5 / 1 = 36.5
S (conc. in gm / litre ) = NE
N = S / E
N = 0.0365 /36.5
N = 0.001 = 10 -3
10 -3 N HCl
[H +] = 10 -3
pH = – log [H +]
= – log [ 10 -3]
pH = – [- 3 log 10 ]
because , log 10 = 1
Therefore,
pH = – [- 3 x 1 ] = 3
pH = 3 Ans.
Question 3) Dissociation constant of a weak acid (HA) is 4.9 x 10-8. For deci normal solution , Calculate –
(a) % ionisation
(b) pH
(c) OH– concentration
Solution )
a) For HA,
K = ∝ 2 /V
K = 4.9 x 10-8
deci normal solution means N/10 or 0.1 N solution,
V = 1 / N = 1 /0.1 = 10 litre
∝ = KV . UNDERROOT
∝ = 4.9 x 10 -8 x 10
∝ = 7 x 10 -4
% of ∝ = 7 x 10 -4 x 100 =7 x 10 -2 = 0.07 %
% of ∝ = 0.07 % Ans.
b) [H+] = ∝ / V
= 7 x 10 -4 / 10 = 7 x 10 -5
[H+] = 7 x 10 -5
pH = – log [H+]
= – log [ 7 x 10-5]
[log a x b = log a + log b]
so,
pH = – [log 7 + log 10 -5]
[log ab = b log a]
= – [ 0.8451 – 5 log 10]
because , log 10 = 1
p H =- [ 0.8451 – 5 x 1]
Therefore,
pH = – 0.8451 + 5 x 1 ] = 4.1549
pH = 4.1549 Ans.
c) [OH –] = ?
[H +] [OH –] = 10 -14
[OH –] = 10-14 / [H +]
[OH –]= 10 -14/ 7 x 10 -5 = 0.143 x 10-9
[OH –]= 1.43 x 10 -10 Ans.
Question 4) Calculate the pH of solution obtained by mixing 10 ml of 0.1 M HCl and 40 ml of 0.2 M H2SO4 ?
Solution )
V of HCl = 10 ml
0.1 M HCl = 0.1 N HCl (because HCl is mono basic acid)
Milli equivalents of [H+] from HCl = NV(ml)
= 0.1 x 10 = 1
V of H2SO4 = 40 ml
0.2 M H2SO4 = 0.2 x 2 N H2SO4 = 0.4 N H2SO4 (because H2SO4 is dibasic acid)
Milli equivalents of [H+] from H2SO4 = NV(ml)
= 0.4 x 40 =16
Total Milli equivalents of [H+] in solution =1 + 16 = 17
Total volume = 10 + 40 = 50 ml
[H+] = Milli equivalents / Total volume
= 17 / 50 =3.4 x 10 -1
[H+] = 3.4 x 10-1
pH = – log [H+]
= – log [ 3.4 x 10-1]
[log a x b = log a + log b]
so,
pH = – [log 3.4 + log 10-1]
[log ab = b log a]
= – [ 0.5315 – 1 log 10]
because , log 10 = 1
p H =- [ 0.5315 – 1 x 1]
Therefore,
pH = – 0.5351 + 1 ] = 0.4649
pH = 0.4649 Ans.
Question 5 ) Calculate the pH(Power of hydrogen ion )of a solution which contains 100 ml of 0.1 M HCl and 9.9 ml of 1.0 M NaOH ?
Solution )
V of HCl = 100 ml
0.1 M HCl = 0.1 N HCl (because HCl is mono basic acid)
Milli equivalents of [H+] from HCl = NV(ml)
= 0.1 x 100 =10
V of NaOH = 9.9 ml
1.0 M NaOH = 1.0 N NaOH (because NaOH is mono acidic base)
Milli equivalents of [OH–] from NaOH = NV(ml)
= 1.0 x 9.9 = 9.9
Milli equivalents of HCl left in solution =10 – 9.9 = 0.1
Total volume = 100 + 9.9 = 109.9 ml
[H+] = Milli equivalents / Total volume
= 0.1 / 109.9 = 0.000909
[H+] = 9.09 x 10 -4
pH = – log [H+]
= – log [ 9.09 x 10-4]
[log a x b = log a + log b]
so,
pH = – [log 9.09 + log 10-4]
[log ab = b log a]
= – [ 0.9586 – 4 log 10]
because , log 10 = 1
p H =- [ 0.9586- 4 x 1]
Therefore,
pH = – 0.9586 + 4 ] = 3.0414
pH = 3.0414 Ans.
Question 6) Calculate the pH of a solution at 250C which is twice as alkaline as pure water ?
Solution )
At 250 C,
[OH–] = 2 x [OH –] of water
In water, [H + ] = [OH–]= 10 -7
[OH –] = 2.0 x 10 -7
pOH = – log [OH –]
= – log [ 2.0 x 10-7]
[log a x b = log a + log b]
so,
pOH = – [log 2 + log 10-7]
[log ab = b log a]
= – [ 0.3010 – 7 log 10]
because , log 10 = 1
pOH =- [ 0.3010 – 7 x 1]
Therefore,
pOH = – 0.3010 + 7 x 1 ] = 6.6990
pH + pOH = 14
pH = 14 -pOH
= 14 – 6.6990
pH = 7.3010 Ans.