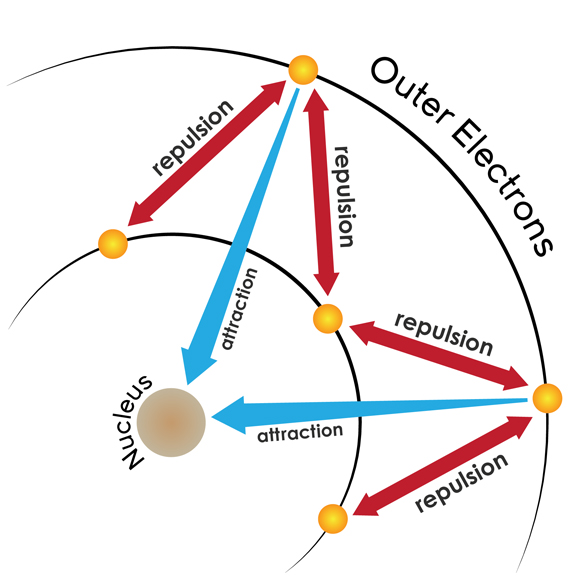
Screening factor or Screening effect constant-

source : wikipedia
Screening factor or Screening effect constant-
When we consider the attraction of the nucleus over an electron , there are always some electrons in between these two. These electrons partially neutralise the effect of attraction and thus the effective nuclear charge for an electron is much less than the actual nuclear charge . This screening of inner electron produces the screening factor . It is represented by ‘σ’ . It is related to effective nuclear charge by the following relation-
Effective nuclear charge = Actual nuclear charge – σ
Zeff = Z – σ
Rules to calculate Screening effect-
The most accepted method for the calculation or determination of the value of screening factor is given by Slater. There are some rules to find screening factor . According to Slater,
1) Each electron of the orbit higher than the electron under consideration contributes zero.
2) Each electron of the same orbit contributes 0.35 except ‘1s ‘ electron which contributes 0.30.
3) Electron of (n-1) orbit contributes 0.85.
4) All the electrons in the (n-2 ) shell contributes 1.0 .
Ex –
i) 3Li – 1s2,2s1
Screening factor ‘σ ‘ = 2 x 0.85 = 1.7 Ans.
Zeff = Z – σ
Z = 3
Z eff = 3 – 1.7 = 1.3 Ans.
ii) 4 Be – 1s2,2s2
Screening factor ‘σ ‘ = 1 x 0.35 + 2 x 0.85 ‘
σ = 1 x 0.35 + 2 x 0.85
σ = 0.35 + 1.7 = 2.05 Ans.
Zeff = Z – σ
Z = 4
Z eff = 4.0 – 2.05 = 1.95 Ans.
iii) 5 B – 1s2 , 2s2 , 2p1
Screening factor ‘σ ‘ = 2 x 0.35 + 2 x 0.85 ‘
σ = 2 x 0.35 + 2 x 0.85
σ = 0.70 + 1.7 = 2.4 Ans.
Zeff = Z – σ
Z = 5
Z eff = 5.0 – 2.4 = 2.6 Ans.
iv) 6 C – 1s2 , 2s2 , 2p2
Screening factor ‘σ ‘ = 3 x 0.35 + 2 x 0.85 ‘
σ = 3 x 0.35 + 2 x 0.85
σ = 1.05 + 1.7 = 2.75 Ans.
Zeff = Z – σ
Z = 6
Z eff = 6.0 – 2.75 = 3.25 Ans.
v) 7 N – 1s2 , 2s2 , 2p3
Screening factor ‘σ ‘ = 4 x 0.35 + 2 x 0.85 ‘
σ = 4 x 0.35 + 2 x 0.85
σ = 1.40 + 1.7 = 3.10 Ans.
Zeff = Z – σ
Z = 7
Z eff = 7.0 – 3.10 = 3.90 Ans.
vi) 8 O – 1s2 , 2s2 , 2p4
Screening factor ‘σ ‘ = 5 x 0.35 + 2 x 0.85 ‘
σ = 5 x 0.35 + 2 x 0.85
σ = 1.75 + 1.7 = 3.45 Ans.
Zeff = Z – σ
Z = 8
Z eff = 8.0 – 3.45 = 4.55 Ans.
vii) 9 F – 1s2 , 2s2 , 2p5
Screening factor ‘σ ‘ = 6 x 0.35 + 2 x 0.85 ‘
σ = 6 x 0.35 + 2 x 0.85
σ = 2.10 + 1.7 = 3.80 Ans.
Zeff = Z – σ
Z = 9
Z eff = 9.0 – 3.80 = 5.20 Ans.
viii) 10 Ne – 1s2 , 2s2 , 2p6
Screening factor ‘σ ‘ = 7 x 0.35 + 2 x 0.85 ‘
σ = 7 x 0.35 + 2 x 0.85
σ = 2.45 + 1.7 = 4.15 Ans.
Zeff = Z – σ
Z = 10
Z eff = 10.0 – 4.15 = 5.85 Ans.
ix) 11 Na – 1s2 , 2s2 , 2p6 , 3s1
Screening factor ‘σ ‘ = 8 x 0.85 + 2 x 1.0 ‘
σ = 8 x 0.85 + 2 x 1.0
σ = 6.80 + 2.0 = 8.80 Ans.
Zeff = Z – σ
Z = 11
Z eff = 11.0 – 8.80 = 2.20 Ans.
x) 19 K – 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s1
Screening factor ‘σ ‘ = 8 x 0.85 + 10 x 1 ‘
σ = 6.80 + 10 = 16.80 Ans.
Zeff = Z – σ
Z = 19
Z eff = 19.0 – 16.80 = 2.20 Ans.
xi) 20 Ca- 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2
Screening factor ‘σ ‘ = 1 x 0.35 + 8 x 0.85 + 10 x 1 ‘
σ = 0.35 + 6.80 + 10 = 17.15 Ans.
Zeff = Z – σ
Z = 20
Z eff = 20.0 – 17.15 = 2.85 Ans.
xii) 30 Zn – 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10
Screening factor ‘σ ‘ = 1 x 0.35 + 18 x 0.85 + 10 x 1 ‘
σ = 0.35 + 15.30 + 10 = 25.65 Ans.
Zeff = Z – σ
Z = 30
Z eff = 30.0 – 25.65 = 4.35 Ans.
xiii) 38 Sr – 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10 ,4p6, 5s2
Screening factor ‘σ ‘ = 1 x 0.35 + 8 x 0.85 + 28 x 1 ‘
σ = 0.35 + 6.80 + 28 = 35.15 Ans.
Zeff = Z – σ
Z = 38
Z eff = 38.0 – 35.15 = 2.85 Ans.
xiv) 55 Cs – 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10 ,4p6, 5s2 , 4d10 ,5p6 , 6s1
Screening factor ‘σ ‘ = 8 x 0.85 + 46 x 1 ‘
σ = 6.80 + 46= 52.80 Ans.
Zeff = Z – σ
Z = 55
Z eff = 55.0 – 52.80 = 2.20 Ans.
xv) 56 Ba – 1s2 , 2s2 , 2p6 , 3s2 , 3p6 , 4s2 , 3d10 ,4p6, 5s2 , 4d10 ,5p6 , 6s2
‘σ ‘ = 1 x 0.35 + 8 x 0.85 + 46 x 1 ‘
σ = 0.35 + 6.80 + 46= 53.15 Ans.
Zeff = Z – σ
Z = 56