VSEPR Theory

source : Slideserve.com
VSEPR Theory [Valence Shell Electron Pair Repulsion Theory] -Applications-
Shapes of molecules containing lone pair and bond pair of electrons-
1) Molecules containing three electron pairs-
a)Molecules containing one lone pair and two bond pair of electrons[AB2L]-
Ex . SO2
In this molecule 3 electron pair ( 2bp and 1 lp) are present.The three bp acquire trigonal planar geometry with bond angle 1200 but one position is occupied by a lone pair , hence the geometry becomes distorted and acquire angular or ‘V’ or bent shape.
lp-bp > bp-bp
Therefore bonded electron pairs are pushed more closer and O- S – O bond angle gets reduced to 119 0 from 1200.

source : Geometry of Molecules
2) Molecules containing four electron pairs-
a)Molecules containing one lone pair and three bond pair(bp) of electrons [AB3L]-
Ex . ammonia molecule-
In ammonia , nitrogen is surrounded by four electron pairs ( 3bond pair and 1lone pair). Due to presence of lone pair , geometry of NH3 becomes distorted tetrahedral ( pyramidal).
lp-bp > bp-bp
Hence lone pair of electrons will repel the bps strongly and bond angle gets reduced to 107 0 from 109028′ and geometry becomes pyramidal.
other Ex. – PCl3 . NF3 , H3O+ etc.

source : Chemical forums.com
b)Molecules containing two lone pair and two bond pair of electrons [AB2L2]-
Ex – H2O
Four pairs of electrons are present around the central oxygen atom. The distortion is due to the presence of 2 lone pair of electrons with 2 bond pairs
lp-lp > lp-bp > bp-bp
Thus in water 2 lone pairs of electrons move away from each other while 2 bond pairs are forced closer to each other . Hence resulting a decrease in H – O – H angle from 109028′ to 104.50. Hence geometry become bent or angular.
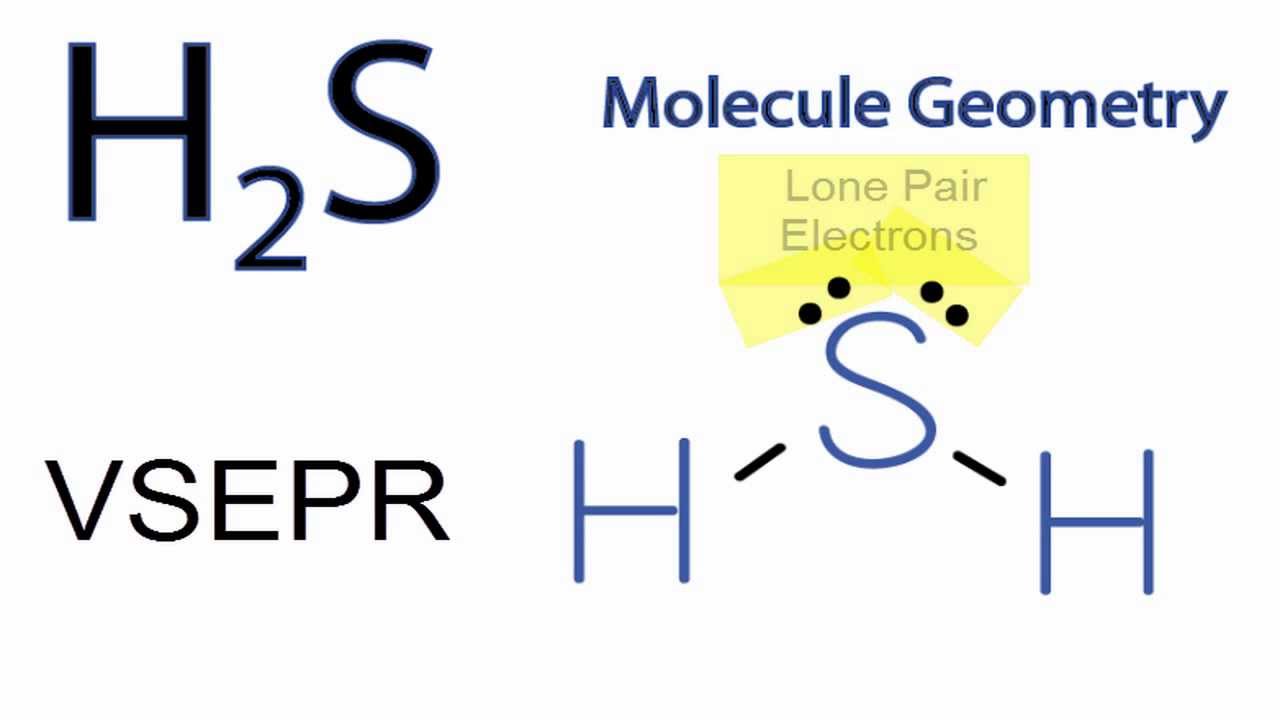
Other Ex. – H2S , F2O ,SCl2
source : en.wikipedia.org