VSEPR Theory

source : Slideplayer.com
VSEPR Theory [Valence Shell Electron Pair Repulsion Theory] -Applications-
1) Molecules containing five electron pairs-
a)Molecules containing one lone pair and four bond pair(bp) of electrons [AB4L]-
Ex – SF4
In SF4 molecule four bond pair and one lone pair (lp) of electrons are present around the central Sulphur atom. lone pair is present in equatorial position . The distortion is due to the presence of lone pair .
lp-bp > bp-bp
Geometry becomes distorted or like see saw. The bond angle in SF4 are 890 and 1170 instead of 900 and 1200 respectively.

source : Wikipedia
b)Molecules containing two lone pair and three bond pair of electrons [AB3L2]-
Ex- ClF3
In ClF3 molecule three bond pair and two lone pair of electrons are present around the central Chlorine atom. Both lone pairs are present in equatorial positions . The distortion is due to the presence of lone pairs .
lp – lp > lp-bp > bp-bp
Geometry becomes ‘ T’ shaped . The bond angle in ClF3 is 87.60 instead of 900.

source : Chemistry Libre texts
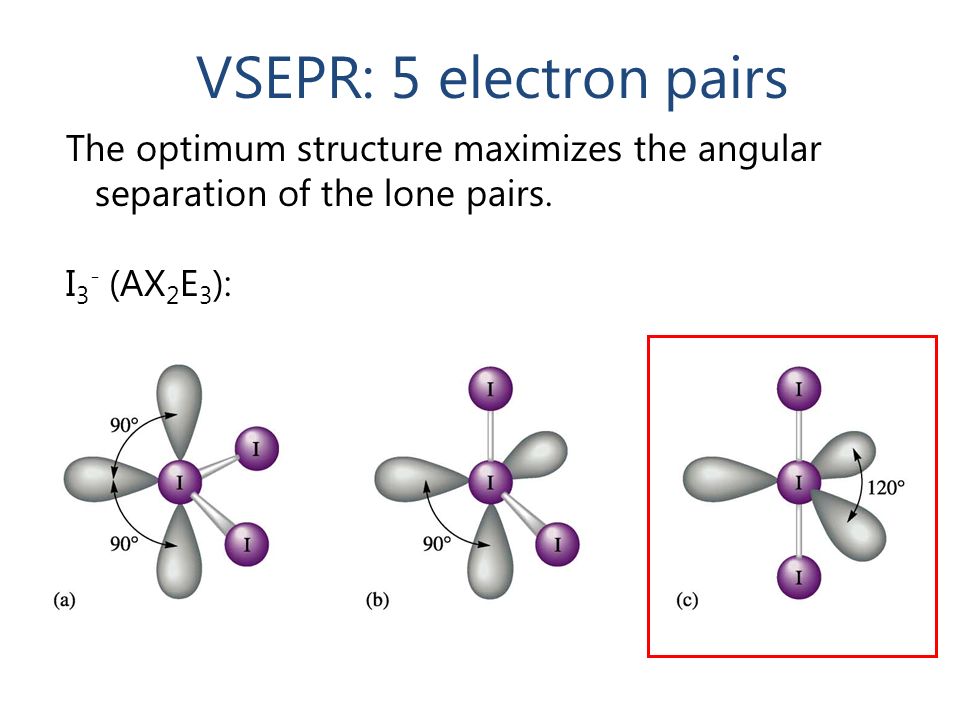
c)Molecules containing three lone pair and two bond pair of electrons [AB2L3]-
Ex – XeF2
In XeF2 molecule two bond pair and three lone pair of electrons are present around the central Xenon atom. Three lone pairs are present in equatorial position . In this case net repulsion on the bonds due to lone pairs is zero .
lp – lp > lp-bp > bp-bp
Geometry becomes linear . The bond angle is 1800.

source : Quora.com
2) Molecules containing Six electron pairs-
a)Molecules containing one lone pair and five bond pair of electrons [AB5L]-
Ex – BrF5
In BrF5 molecule 5 bp and 1 lone pair are present . Out of 6 electron pairs , one position is occupied by a lone pair . Since all the positions in octahedral geometry are equivalent .So lone pair may be placed at any position.
lp-bp > bp-bp
Geometry of BrF5 is square pyramidal.

source : en.wikipedia.org
b)Molecules containing two lone pair and four bond pair of electrons [AB4L2]-
Ex – XeF4
The six electron pairs has octahedral geometry but in XeF4 molecule 4 bp and 2 lone pairs are present.So two positions are occupied by 2 lone pairs . Hence geometry becomes square planar.

source : Slide player.com